Spring 2025 (Volume 35, Number 1)
Year in Review:
Complex (Not So Basic) Science
By May Choi, MD, MPH, FRCPC
Download PDF

In the world of basic and translational science in rheumatology, research papers are becoming increasingly complex. From intricate immune pathways to the advanced technologies used to study our diseases, understanding these findings is a challenge even for seasoned immunologists. Therefore, for my “Year in Review: Basic Science” presentation at the 2025 Canadian Rheumatology Association Annual Scientific Meeting (Calgary, Alberta), I took a different approach. Rather than explaining what were the most significant basic science findings from 2024 related to rheumatology, I focused on how these groundbreaking discoveries were made, particularly in light of key recent trends: artificial intelligence (AI), immune profiling, and multi-omics.
The first key technology that I discussed was the growing role of AI in medicine. Though the term AI was coined in the 1950s, it’s only recently that it has had a major impact on health research, thanks to advances in supercomputing and improved patient data collection, now reaching down to the molecular level. AI has become essential for interpreting and integrating large datasets (big data) to understand disease pathophysiology accurately and efficiently. AI can also assist in revealing new patterns that would be challenging to detect with traditional statistical methods. Machine learning algorithms, a type of AI which includes deep learning, can also analyze data such as images, text, and audio.
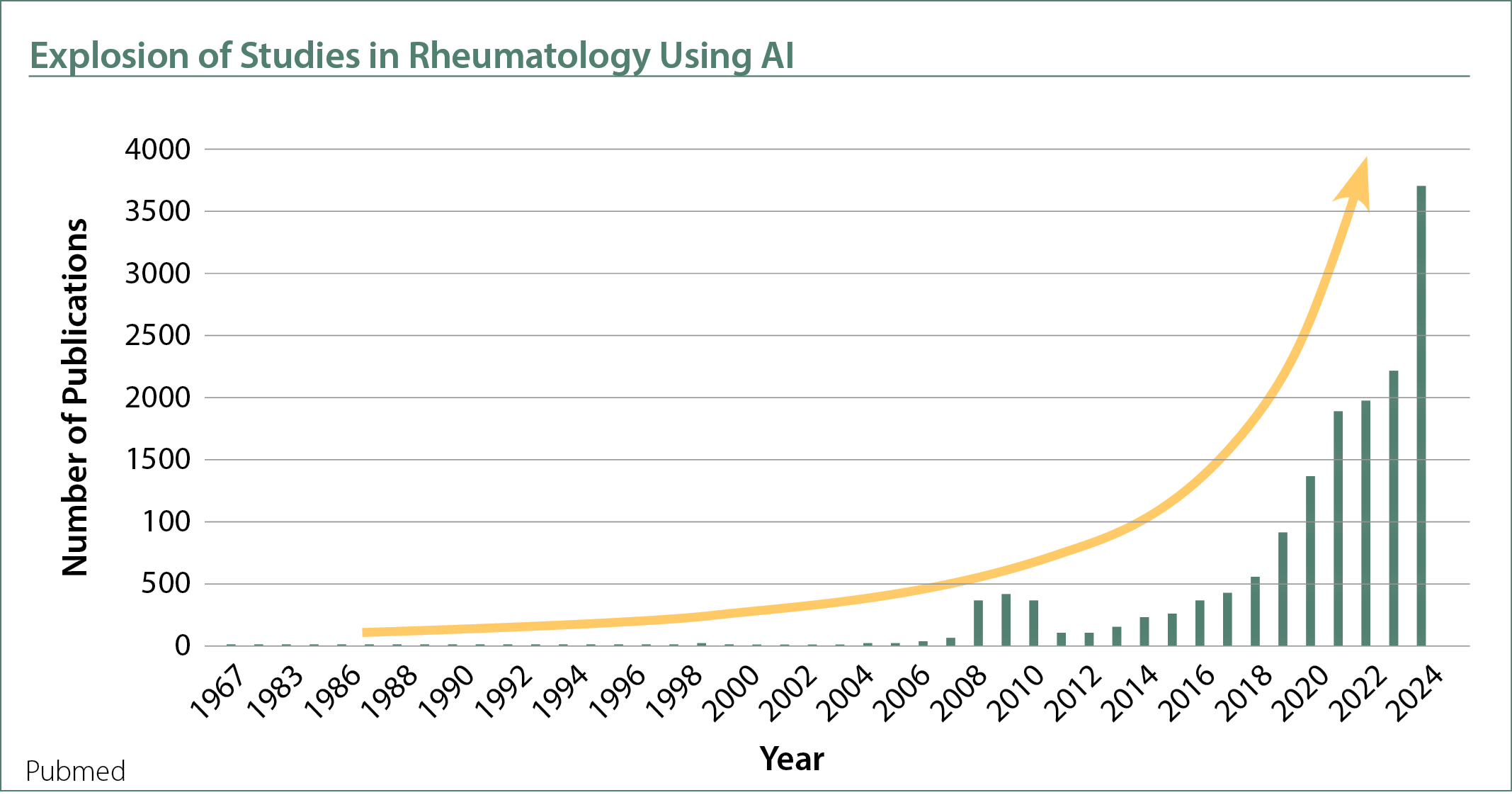
In autoimmune disease research, experts from the National Institutes of Health held a meeting in 2024 that identified three critical areas for future research: disease heterogeneity, natural history, and the role of tissues in disease pathogenesis.1 Biomarkers addressing these areas are key to advancing our understanding of autoimmune diseases, and AI plays a crucial role in identifying these biomarkers. We recently showed in a review of machine learning studies (a type of AI) in lupus, that AI is widely used to analyze biomarker datasets to identify biomarkers that can improve diagnostic accuracy and predict disease complications.2 These biomarkers included immune cell subsets (e.g., T cells, B cells) and multi-omics (e.g., genomics, proteomics, lipidomics). This combination of AI with immunophenotyping and multi-omics has become a popular approach in basic science to study disease pathogenesis in our field.
A typical workflow integrating AI, immunophenotyping, and multi-omics starts with collecting biospecimens such as blood or tissue samples. These samples are then analyzed to determine immune cell subtypes (e.g, flow or mass cytometry) and various -omics (e.g., using whole genome sequencing, single-cell RNA sequencing, mass spectrometry). The resulting data typically form a large biomarker dataset that is analyzed by machine learning algorithms to identify patterns and potential biomarkers for patient stratification, diagnosis, prognosis, disease pathogenesis, and novel targets for therapy.
Looking to the near future, there’s a drive for even larger AI models capable of processing more parameters and connections between their “neurons”. For context, the human brain has about 100 trillion connections, while models like ChatGPT use around 1 trillion connections.3 Given the exponential growth of AI applications, it would not be surprising that AI models will soon get there. We also have other technologies to look forward to, including spatial proteomics, which was named Method of the Year by Nature.4 This technique takes -omics approaches to the next level by providing a spatial map of protein expression within tissues. It may revolutionize how researchers study diseases using tissue biopsies—such as those from the synovium, muscle, kidneys, or skin—providing a comprehensive view of the immune system. After all, a picture is worth a thousand words! This method offers the potential for more personalized treatments and deeper insights into disease mechanisms, opening new avenues for research and improving patient care in rheumatology.
May Y. Choi, MD, MPH, FRCPC
Associate Professor,
Cumming School of Medicine
University of Calgary and
Alberta Health Services
Calgary, Alberta
References:
1. Guerau-de-Arellano M, Morris MA, Sherman MA, Esch TR. Meeting report: Hidden links in autoimmunity. Science Immunology?. 2024;9(102):eads5884.
2. Zhan K, Buhler KA, Chen IY, Fritzler MJ, Choi MY. Systemic lupus in the era of machine learning medicine. Lupus Science & Medicine. 2024;11(1):e001140.
3. Ananthaswamy A. In AI, is bigger always better? Nature. 2023;615(7951):202-5.
4. Karimi E, Simo N, Milet N, TE W, ALSH A, QU N, et al. Method of the Year 2024: spatial proteomics. Nat Methods. 2024;21:2195-6.
|
