Fall 2022 (Volume 32, Number 3)
Survey Results:
CRA Choosing Wisely —
Ordering RF & ACPA Tests and Monitoring DMARDs
Download PDF
This issue’s Joint Count survey, in collaboration with
the CRA Choosing Wisely submcommittee, aimed
to better understand when rheumatoid factor (RF)
and anti-citrullinated protein antibody (ACPA) tests are
ordered, and how disease-modifying anti-rheumatic drugs
(DMARDs) are monitored. The CRA Choosing Wisely
subcommittee (at the time of this writing) plans to publish
new statements regarding the ordering of RF and ACPA
tests, as well as the monitoring of DMARDs this fall (these
statements are shown in the box below). The survey was
sent to members of the CRA (603 members), and a total of
68 responses were received.
The first question of the survey queried members
about how often they monitored lab work in a stable patient
with inflammatory arthritis on non-biologic disease-modifying
anti-rheumatic drug therapy. Most (approximately
70%) indicated every three months, while 18% said
every 2 months; 9% said less often than every 3 months;
and only 3% said every month. One respondent commented
that the question was too vague, and that patients’
comorbidities would also need to be taken into account.
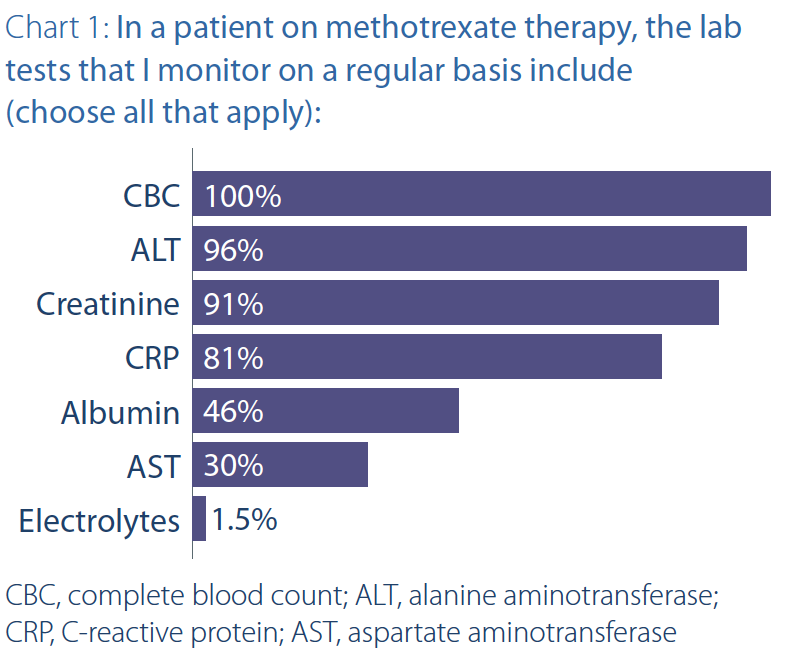
The next question asked members about what tests
they monitor for patients on methotrexate therapy. Responses
are shown in Chart 1. A complete blood count
(CBC) and alanine aminotransferase (ALT) were almost
universal. Overall, there seems to be a lot of variability in
what tests are ordered and how often. It should be noted
that some provinces, such as Ontario, limit the ordering
of aspartate aminotransferase (AST) to GI specialists.
As well, with the current health human resources crisis
affecting laboratory medicine, physicians have been requested
to review their routine lab ordering protocols,
especially for non-specific tests such as ESR.
For the next question, only 26% of survey respondents
were aware that the symptoms located in the metatarsophalangeal
(MTP) joints are not part of the EULAR definition of
clinically suspicious arthralgia (CSA) at risk of developing
rheumatoid arthritis (RA). The EULAR definition of CSA includes
symptoms of new joint pain, pain in metacarpophalangeal
(MCP) joints, morning stiffness >60 minutes, most
severe symptoms in the morning, presence of 1st-degree
relative with RA, difficulty making a fist and positive MCP
squeeze test.
Regarding the last question, the results reflected that
only about a quarter of respondents were aware that among
individuals with clinically significant arthralgia with positive
RF and ACPA, 30-60% will never develop RA (for more
information visit rheum.ca/resources/choosing-wisely).
The CRA would love to hear your reflections. For any
feedback on the survey, please reach out to Mona Bosinceanu
at mbosinceanu@rheum.ca. For further information, visit choosingwiselycanada.org or rheum.ca/resources/choosing-wisely/.
|
Two New Choosing Wisely Statements:
|
RF & ACPA Tests:
Do not order Rheumatoid Factor (RF) and Anti-Citrullinated Protein Antibody (ACPA) tests unless patients have clinically
suspicious arthralgia (CSA*) or arthritis on exam.
DMARD Monitoring:
Do not order labs for drug toxicity monitoring (i.e., CBC, liver enzymes, creatinine) more often than every 8-12 weeks
for patients on a stable dose of non-biologic disease modifying anti-rheumatic drugs (DMARDs), in patients without
comorbidities or (baseline) lab abnormalities.
*EULAR defined characteristics defining Clinically Suspect Arthralgia at risk for RA
|
The CRA Choosing Wisely Subcommittee would like to thank
the CW working group: Maryam Obaidalla (Ontario);
Bindu Nair (Saskatchewan); Nicole Beckett (Nova Scotia)
Nicolas Richard (Quebec); Zachary Shaff (Nova Scotia)
Nadia Lucia (Alberta); and Shirley Lake (Ontario)
|




