Summer 2021 (Volume 31, Number 2)
A Brief History of Treating
Rheumatoid Arthritis
By Reza Mirza, MD, (based on a discussion with
Dr. Arthur Bookman)
Download PDF
"One of the most intractable, obstinate, and crippling diseases that can befall the human body."
– Lane and Griffiths, 1890
"Cases of ruin and despair, in one sense more malignant than cancer."
– Spender, 1889
 
Dr. Jacques Forestier Dr. Henri Forestier
1920s:
“All that is gold does not glitter.”
– J.R.R. Tolkien
In 1929, Dr. Jacques Forestier—son
of Henri, the founder of La Ligue Internationale Contre Le Rheumatisme—posited
that rheumatoid arthritis (RA)
and tuberculosis (TB) shared similar
features: febrile illness with leukocytosis,
anemia, and general malaise.
He hypothesized that given gold’s
usefulness in TB, perhaps it would
prove useful in RA.
Over the next several years, he published a number of
case series of gold trials in The Lancet. He injected 250 mg
of gold thiopropanol intramuscular (IM) weekly x 10-12,
waited a month, and in some cases gave another course.
Five of 15 patients had “excellent” response; another
five had “much improved,” two had “minimal response,”
and three were no worse. For comparison, we typically
cite biologic response rates at 20% for ACR70, and 40%
for ACR50+ (I say plus because people like myself forget
ACR50 includes ACR70).
There remained ongoing controversary as to whether gold
worked, until 1945, when Thomas Fraser published the results
of the first double-blind randomized clinical trial (RCT)
of any anti-rheumatic drug. It compared gold to placebo. He wasn’t fortunate enough to have the
Clinical Disease Activity Index (CDAI)
or American College of Rheumatology
(ACR) scoring system. He admitted
himself: “It is difficult to decide what
criteria to use.” Forty-two percent
(42%) had great improvement based on
his impression.
In the 1980s, oral gold was developed:
More convenient but less effective.
Mechanism of action (of gold):
- Patients treated with gold have decreased
immunoglobulins, rheumatoid factor,
and circulating immune complexes.
- Gold can dissociate antigenic peptides from MHCII,
decreasing antigen presentation, demonstrated
in vivo on HLA-DRB1 (the shared epitope).
- Gold blocks prostaglandin E2 production.
1940-50s: Rx. ASA 325 mg 3 tablets QID—You read
that right!
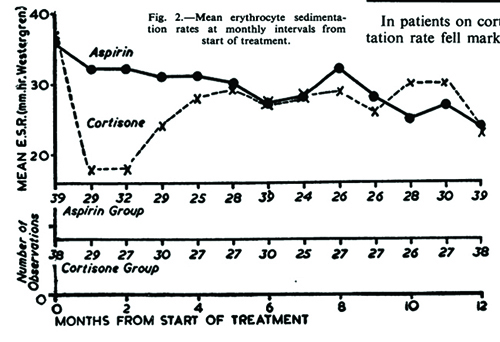
The Empire Rheumatism Trial (1955) was the CYCLOPS
trial of its day.1 It proved acetylsalicylic acid (ASA) was no
different than cortisone in terms of improvements in joint
count and erythrocyte sedimentation rate (ESR) and ushered
in an era of proliferating nonsteroidal anti-inflammatory
drugs (NSAIDs)!
Enteric-coated ASA
given in increasing
doses until maximally
tolerated. The usual
optimum dose was
975 mg QID (3.9 g
OD). You titrated
to tinnitus then
dropped the dose.
Not the only instance
rheumatologists
invoked such a rule.
Dr. Bookman: “Nobody had
an MI on high-dose aspirin. We
thought rheumatoid protected from
coronary disease until we switched
to ibuprofen and naproxen.”
Salicylate Therapy ‒ Fremont Smith & Bayles
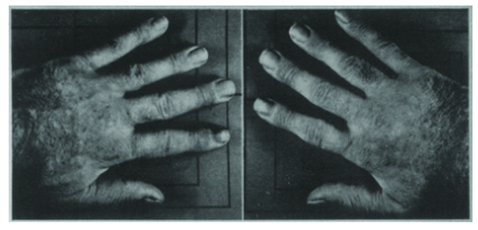
9th day of ASA
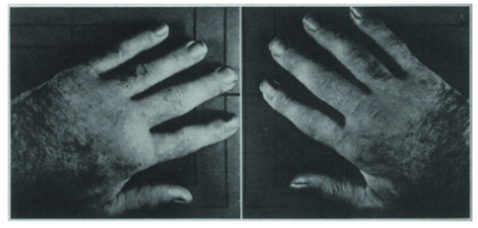
48 hours after
withdrawing ASA
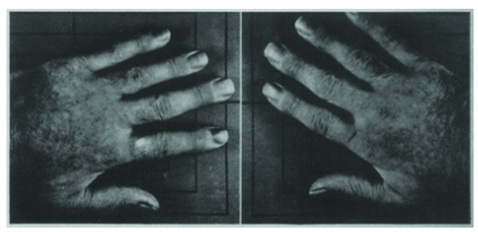
72 hours after
resuming ASA
1950s: Cortisone
The first realization there may be an agent to put RA into remission came when physicians realized patients with RA who became jaundiced underwent spontaneous remission. The hunt was on for “Nature’s Dramatic Antidote”: “Volunteers with rheumatoid arthritis were given bile salts by mouth, a derivative of a bile acid (decholin) orally and intravenously, liver extracts parenterally, ox bile by proctoclysis [per rectum], and large amounts of human bile by stomach tube…" None of these worked!
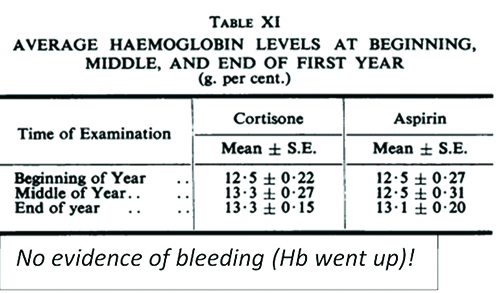
Another clue came from women with RA who dramatically
improved during pregnancy. The focus switched to hormones.
In 1948, Dr. Kendall (a biochemist who isolated thyroxine
and several adrenal hormones including cortisone)
and Dr. Hench of Mayo Clinic trialed “Compound E” (cortisone)
on a patient with rheumatism at a dose of 100 mg IM
daily, and she improved dramatically within three days.
And so, they won the Nobel prize! Dr. Laurence Rubin
insists you read their Nobel lecture on the discovery.2 It is
very good.
The next 60 years introduced the drugs we are familiar
with, so we can leave their tales brief:
1960s: NSAIDs. The first was ibuprofen (patented 1962,
marketed 1969); the second was naproxen (patented 1967,
marketed 1976). At one point there were 15 NSAIDs on the
Canadian market. Heart attack rates shot up. Hospitalizations
for ulcer complications became epidemic.
1970s: Methotrexate and Cyclophosphamide. Rex Hoffmeister,
a practicing rheumatologist from Spokane, Washington,
reported positive effects with intramuscular MTX
in 1972. At the ACR meeting people laughed him off. It
took the stodgy rheumatology community until the 1980s
to do the first double-blind trial.
1990s: Leflunomide received approval in 1998 in the U.S.:
the same year as etanercept.
Conclusion Rheumatology is the specialty with the most patientimportant
advances in the past several decades, as I see it.
My colleagues and I cannot wait for what the future holds.
Only a few beasts await to be tamed: Scleroderma, Sjogren’s
syndrome, the many-faced wolf (SLE), and the vasculitides.
References:
1. de Groot, K, et al. "Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic
antibody—associated vasculitis: a randomized trial." Annals of internal medicine. 2009; 150(10): 670-680.
2. Kendall, E. C. The development of cortisone as a therapeutic agent. Antibiot Chemother (Northfield). 1951;
1(1): 7-15.
Reza Mirza, MD, Rheumatology trainee,
University of Toronto, Toronto, Ontario
The Toronto Wellesley Hospital (1963-1998),
a 40-bed Inpatient Rheumatology Ward:
A Reflection by Dr. Bookman
Patients were brought in from all over Ontario, sometimes
from the back of a barn, many times completely immobile.
Patients would be admitted for several weeks.
They were brought to hospital for physiotherapy, occupational therapy, rehabilitation, medication management,
reconstructive surgery, splints, springs, and slings. Everyday
at noon, physiotherapy was conducted over the intercom and
patients followed along in their beds
There was a heated therapeutic pool. Immobile patients
would be lifted in using a cradle. Hands were dipped in warm
paraffin wax (heated using a double-boiler) to relieve AM
stiffness prior to hand physiotherapy.
Rheumatology trainees would inject several joints at
a time in each patient each day. The only drugs available
were gold, NSAIDs, cortisone, and chloroquine. Chloroquine worked much better than hydroxychloroquine, but
had higher rates of retinal toxicity and also caused corneal
toxicity affecting night vision.
|
