Spring 2021 (Volume 31, Number 1)
MIS-C and PIMS:
The Alphabet Soup of
COVID-associated
Hyperinflammation in Children
By Tala El Tal, MD; and Rae S. M. Yeung, MD, FRCPC, PhD
Download PDF
Patient Case:
An eight-year-old previously healthy South Asian boy presented to the emergency department (ED) with four days of persistent
fever, abdominal pain, vomiting and diarrhea, associated with bilateral non-purulent conjunctivitis, rash over his chest,
lower limbs and palms, and red swollen cracked lips. Four weeks prior to presentation, his father tested positive for severe
acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on nasopharyngeal swab. At the time, the patient was asymptomatic
and was not tested. On arrival to ED, he was hypotensive with a blood pressure of 78/47 mm Hg and heart rate of 150 beats/
min despite receiving 40 mL/kg of fluid. Peripherally, he was cool to touch and had prolonged capillary refill.
Laboratory results on admission were significant for markedly elevated C-reactive protein (CRP), thrombocytopenia,
lymphopenia, hyperferritinemia, hypoalbuminemia, hypertriglyceridemia, elevated liver enzymes, coagulopathy, and
markedly elevated troponin I and
N-terminal-pro-brain natriuretic peptide (NT-proBNP). An echocardiogram (ECHO)
showed reduced left ventricular systolic function and dilated left anterior descending artery. An electrocardiography
(ECG) showed diffuse non-specific T-wave abnormalities. The patient’s nasopharyngeal swab for SARS-CoV-2 was indeterminate
on repeated polymerase chain reaction (PCR) testing, but serology testing for COVID-19 IgG antibody was reactive.
He was diagnosed with multisystem inflammatory system in children (MIS-C), also known as pediatric inflammatory
multisystem syndrome (PIMS) temporally associated with SARS-CoV-2 and admitted to the intensive care unit (ICU) where
he required inotropic support for his cardiac dysfunction. He was given IVIG and steroids as immunosuppressive agents
to control his hyperinflammation together with anti-platelet doses of ASA. He improved dramatically requiring only a
4-day hospital stay with the first two in the ICU. He was discharged on a three-week course of weaning steroids with full
recovery and no long-term adverse cardiovascular consequences.
At the start of the COVID-19 pandemic, it was
thought that most children were either asymptomatic
or had mild disease manifestations. Beginning in
April 2020, clinicians at COVID-19 epicenters observed
the emergence of clusters of school-aged children with fever
and features of Kawasaki Disease (KD) and toxic shock
syndrome (TSS) following COVID-19 in their communities.
Alerts were issued to the medical community and various
different names and case definitions were proposed (visit
cps.ca/en/documents/position/pims for more information).1
For the purpose of this article, the term MIS-C will be used.
This brief update will focus on three practical questions:
- When to suspect MIS-C?
- How to approach the diagnostic evaluation of MIS-C?
- How to treat MIS-C?
When to suspect MIS-C?
The signs and symptoms of MIS-C can largely overlap with
Kawasaki Disease and toxic shock syndrome (TSS). KD is
a hyperinflammatory syndrome presenting as acute multisystem
vasculitis affecting young children. The principal
features include: (1) bilateral conjunctival injection; (2)
polymorphous skin rash; (3) erythema and edema of the
hands and/or feet; (4) cervical lymphadenopathy; and (5)
oral mucosal changes, in the presence of at least 5 days
of fever. KD is known to have a predilection for the coronary
arteries, leading to aneurysm formation in 25% of
untreated cases.2
Similarly, children with MIS-C present with persistent fevers
and multi-organ dysfunction (cardiac, hematologic, gastrointestinal,
neurological, renal, and/or dermatologic) usually
3-6 weeks following prior SARS-COV-2 exposure,3,4
suggesting post-infectious hyperinflammation underlying
the pathobiology.5 Like KD, MIS-C is a syndrome complex
with a wide spectrum of clinical phenotypes. A spectrum
of COVID-19 associated hyperinflammation syndromes
has been proposed6,7 with three clinical patterns along the
hyperinflammation spectrum in MIS-C: Shock, KD, and
fever with inflammation, reflecting the continuum of disease
severity. Early reports were notable for myocarditis, myocardial dysfunction and overt shock requiring inotropic
support as prominent clinical features. Some patients
developed coronary aneurysms, as well as macrophage activation
syndrome (MAS). It was also observed that MIS-C
typically affects healthy children and disproportionately
affects non-Caucasian children, with children from African,
Hispanic and South Asian ethnicity being more affected.
It remains unclear the contribution of environment
versus genetics, with higher rates of COVID-19 noted in
affected communities.
How to approach the diagnostic evaluation of
MIS-C?
A high-index of suspicion for the diagnosis of MIS-C is needed
in children living in COVID-19 hotspots, who present
with prolonged fever and clinical and laboratory features
of inflammation. MIS-C is usually preceded by known
SARS-CoV-2 infection in the child or a family member
several weeks before presentation. Children may present
with features of KD and/or TSS, and often abdominal pain
and other gastrointestinal features are prominent. Of note,
MIS-C is a diagnosis of exclusion and other causes of febrile
illness in children, including other infectious and non-infectious
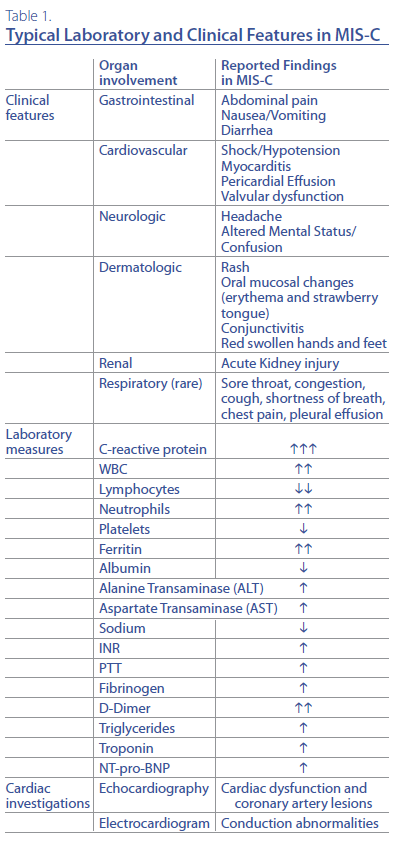
etiologies need to be pursued. Table 1 summarizes
the typical laboratory and clinical findings reported
in MIS-C. Patients have evidence of a hyperinflammatory
state, manifested in laboratory findings of markedly elevated
CRP, and measures compatible with viral infection
(lymphopenia) and MAS including thrombocytopenia and
elevated serum ferritin,6 which together with hyponatremia,
elevated troponin and NT-pro-BNP, are among the
worrisome laboratory findings suggestive of a more severe
disease phenotype.8
How to treat MIS-C?
Although there is rapidly growing literature on MIS-C, management
has been largely based on extrapolated knowledge
from KD treatment. Several groups have convened
expert panels to develop guidance including the American
College of Rheumatology (ACR), which developed guidelines
for the evaluation and treatment of MIS-C.8 Children
admitted to hospital with MIS-C should be managed
by a multi-disciplinary team including rheumatology,
cardiology and other subspecialties as needed. The cornerstone
of therapy is immunomodulation. Treatment recommended
for all children requiring hospitalization for
MIS-C involves step-wise progression of immunosuppression,
starting with high-dose IVIG (2 g per kg per dose)
as first-line therapy. Adjunctive therapy with low-moderate
dose glucocorticoid therapy (prednisone 1-2 mg/kg/d) is
recommended in patients with severe disease, at high-risk
for poor coronary outcome, or as therapy for IVIG failure.
In patients who present with critical organ involvement requiring
inotropic support, or those who are recalcitrant
to IVIG and low-moderate dose steroids, high-dose, pulse
glucocorticoids (10-30 mg/kg/d) are recommended. IL-1
blockers, such as Anakinra (> 4 mg/kg/d), may be considered
in those with disease refractory to IVIG and steroid
therapy, as well as those with features of MAS. Close follow-
up with serial laboratory and cardiac assessment will
help guide duration and tapering of immunosuppression,
with a typical steroid wean over a minimum of 2-3 weeks,
and often longer given the high rate of rebound inflammation
with quicker tapers.8 Other immunomodulatory treatments
have been used and reported in the literature including tocilizumab (IL-6 inhibitor) and infliximab (TNF
inhibitor)9,10 but insufficient data exists for clear recommendations.
Similar to KD, MIS-C patients are treated with
anti-platelet low dose aspirin (ASA) (3-5 mg per kg per day)
as thromboprophylaxis. Anticoagulation with enoxaparin
should be considered in MIS-C patients with coronary artery
aneurysms as per KD management guidelines and in
those with moderate-severe left ventricular dysfunction
(Ejection Fraction < 35%).8
Serial monitoring of clinical and laboratory parameters,
including ECG and ECHO, are recommended as part of the
comprehensive follow up post-discharge.
In summary, MIS-C is a post-infectious hyperinflammatory
syndrome temporally associated with SARS-CoV-2
infections affecting children. There is a wide spectrum of
disease with many sharing features with KD and the most
severely affected children presenting with cardiogenic
shock and MAS. Immunomodulation is the foundation of
therapeutic management, with most children responding
rapidly to treatment. MIS-C remains a rare complication of
SARS-CoV-2 infection.
Rae S. M. Yeung, MD, FRCPC, PhD
Hak-Ming and Deborah Chiu Chair in
Paediatric Translational Research
Professor of Paediatrics, Immunology & Medical Science
University of Toronto
Senior Scientist and Staff Rheumatologist,
The Hospital for Sick Children
Toronto, Ontario
Tala El Tal, MD
Division of Rheumatology
The Hospital for Sick Children
Toronto, Ontario
References and Suggested Readings:
1. Berard RA, Scuccimarri R, Haddad EM, et al Paediatric inflammatory multisystem syndrome temporally
associated with COVID-19. Ottawa: Canadian Paediatric Society; 2020 July 6. Available at
www.cps.ca/en/documents/position/pims. Accessed February 2021.
2. McCrindle BW, Rowley AH, Newburger JW, et al. Diagnosis, treatment, and long-term management
of Kawasaki disease: a scientific statement for health professionals from the American
Heart Association. Circulation. 2017; 135(17):e927-e999.
3. Soma VL, Shust GF, Ratner AJ. Multisystem inflammatory syndrome in children. Curr Opin Pediatr.
2021; 33(1):152-158. doi: 10.1097/MOP.0000000000000974. PMID: 33278107.
4. Kabeerdoss J, Pilania RK, Karkhele R, et al. Severe COVID-19, multisystem inflammatory syndrome
in children, and Kawasaki disease: immunological mechanisms, clinical manifestations and management.
Rheumatol Int. 2021; 41:19–32. https://doi.org/10.1007/s00296-020-04749-4.
5. Henderson LA, Yeung RSM. MIS-C: early lessons from immune profiling. Nat Rev Rheumatol.
2021; 17:75–76. https://doi.org/10.1038/s41584-020-00566-y.
6. Ahmed M, Advani S, Moreira A, et al. Multisystem inflammatory syndrome in children: A systematic
review. EClinicalMedicine. 2020; 26:100527. doi: 10.1016/j.eclinm.2020.100527. Epub
2020 Sep 4. PMID: 32923992; PMCID: PMC7473262.9.
7. Yeung RS, Ferguson PJ. Is multisystem inflammatory syndrome in children on the Kawasaki
syndrome spectrum? J Clin Invest. 2020; 130(11):5681-5684. doi: 10.1172/JCI141718. PMID:
32730226; PMCID: PMC7598074.
8. Henderson LA, Canna SW, Friedman KG, et al. American College of Rheumatology Clinical Guidance
for Multisystem Inflammatory Syndrome in Children Associated With SARS-CoV-2 and
Hyperinflammation in Pediatric COVID-19: Version 1. Arthritis Rheumatol. 2020; 72(11):1791-
1805. doi: 10.1002/art.41454. Epub 2020 Oct 3. PMID: 32705809; PMCID: PMC7405113.
9. Feldstein LR, Rose EB, Horwitz SM, et al. Overcoming COVID-19 Investigators; CDC COVID-19
Response Team. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N Engl J Med. 2020; 383(4):334-346. doi: 10.1056/NEJMoa2021680. Epub 2020 Jun 29. PMID:
32598831; PMCID: PMC7346765.
10. Whittaker E, Bamford A, Kenny J, et al. Clinical Characteristics of 58 Children With a Pediatric Inflammatory Multisystem Syndrome Temporally Associated With SARS-CoV-2. JAMA. 2020; 324(3):259-269. doi:10.1001/jama.2020.10369
|




