Summer 2020 (Volume 30, Number 2)
Joint Count Survey Results:
HCQ and the Risk of Cardiac Toxicity
Download PDF
Amid discussions and controversies surrounding hydroxychloroquine
(HCQ) and the risk of torsades de
pointes, this Joint Count survey, conducted in January
2020 prior to the COVID-19 pandemic, focused on
finding out the perspectives of CRA members on the topic
of potential cardiac toxicity of HCQ. The response rate to
the survey was 95 out of a possible 500, equating to 19%.
More than half of respondents (53%) were academic rheumatologists,
with another 39% in community practice and
8% in both. Four respondents specified that they were in
residency, and two were fellows.
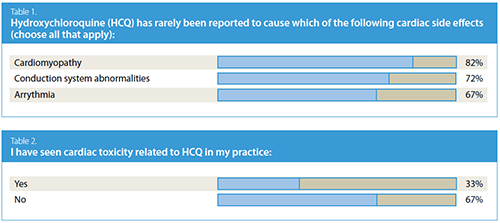
The first question asked members the following: “Hydroxychloroquine
(HCQ) has rarely been reported to cause
which of the following cardiac side effects (choose all that
apply).” Cardiomyopathy was selected by 82% of respondents;
conduction system abnormalities was selected by
72%; and arrhythmia by 67% (see Table 1).
When asked what tests they ordered before starting
HCQ, only nine selected a resting ECG and a single person
selected an echocardiogram.
Finally, when asked whether they have seen cardiac
toxicity related to HCQ in their practice, 33% of respondents
answered affirmatively, while 67% said that they
had not (see Table 2).
While there may be varying perspectives between other
specialists and rheumatologists with regard to HCQ and the
risk of cardiac toxicity, it seems that most rheumatologists
agree that this is a rare risk and that the benefits of HCQ
therapy far outweigh the risks. If you have any additional
feedback for the CRA, please contact Kevin Baijnauth at
kbaijnauth@rheum.ca. A commentary by Dr. Zahi Touma is also available in this issue. Click here to read the article.
|




