Fall 2019 (Volume 29, Number 3)
Assessing Canadian Practice Patterns Regarding Idiopathic Aortitis:
A Qualitative Study
By Marissa Keenan, MD, MSc; Nataliya Milman, MD, FRCPC, MSc; and the Canadian Vasculitis Network
Download PDF
Abstract
Objectives – The purpose of this study was to determine current practices of Canadian rheumatologists with respect to two poorly defined conditions: idiopathic aortitis (IA) and isolated aortitis (IsA).
Methods – An online survey was administered to members of the Canadian Rheumatology Association (CRA) using FluidSurveys™ (www.fluidsurveys.com) in June 2016.
Results – Sixty-eight of the 420 members of the CRA (16%) took the survey. Most (60%) reported seeing one or fewer cases of IA per year, while 23% (15/66) had never seen a case. Twelve participants (26%) reported making a distinction between IA and IsA. Only 38% of participants routinely performed full imaging of chest and abdominal aortic branches during initial assessments. Approach to management was variable. Participants were more likely to treat (with corticosteroids) aortitis with branch vessel involvement compared to IsA. When faced with an asymptomatic patient with normal inflammatory markers, participants were most likely to treat histologically-confirmed aortitis with branch vessel involvement (61%). Only 2/38 respondents felt “perfectly comfortable” managing patients with these conditions.
Conclusions – IA is rare, resulting in lack of familiarity and variability in practices. Further research is needed to close knowledge gaps and facilitate development of informed recommendations.
Introduction
Aortitis is a broad term used to describe disorders characterized by inflammation in the aorta.1 Aortitis can be caused by infectious etiologies or a variety of systemic inflammatory conditions.1 Infectious causes include Salmonella, Staphylococcus, Streptococcus pneumonia, Treponema pallidum, and Mycobacterium tuberculosis.1 Systemic inflammatory conditions associated with aortitis include giant cell arteritis (GCA), Takayasu’s arteritis, Behçet’s disease, Cogan’s syndrome, granulomatosis with polyangiitis, Kawasaki disease, polyarteritis nodosum, polymyalgia rheumatica, relapsing polychondritis, rheumatoid arthritis, sarcoidosis, Sjogren syndrome, HLA-B27 associated spondyloarthropathies, and systemic lupus erythematosus.1 Occasionally aortitis is diagnosed in patients without evidence of systemic disease or infectious etiology; this is generally referred to as idiopathic aortitis (IA). In most patients such aortitis is diagnosed radiologically, most often with computed tomography (CT) or magnetic resonance imaging (MRI). In addition, an increasing number of cases are diagnosed when pathologic review of surgical specimens from resected aortic aneurysms shows features of aortitis.2-4
IA is not a well-defined condition. No specific pathological or clinical criteria exist for its classification or diagnosis, except for the presence of aortic inflammation and the absence of clinical features of another systemic condition, as described above. Current understanding of IA mostly comes from retrospective studies of patients with aortitis diagnosed pathologically,2-6 including a recent series from our institution of 47 cases of aortitis, 32 of which were classified as IA.6
The term “isolated aortitis” (IsA) refers to a specific type of IA where pathology is confined to the aorta and does not involve aortic branch vessels. The terms “idiopathic” and “isolated” aortitis are often used interchangeably in published literature, and few of the published case reports describe imaging findings beyond the culprit area to allow precise characterization. “Isolated aortitis” was added to the recent 2012 Chapel Hill Consensus Conference Nomenclature of Vasculitides8 under the category of “Single-organ vasculitis,” but no specific definition of this condition was suggested by the Chapel Hill nomenclature. Currently there are no guidelines to direct initial workup, treatment, and subsequent monitoring of patients with either IA or IsA, resulting in great case-to-case variability. A strong need exists for systematic studies of idiopathic and isolated aortitis, with the ultimate goal of developing guidelines to standardize management of affected patients. The purpose of this study was to determine the current practice patterns of Canadian rheumatologists with respect to patients with IA and IsA.
Methods: Survey design and administration
The study consisted of a survey administered to members of the Canadian Rheumatology Association (CRA) using the online platform FluidSurveys™ (www.fluidsurveys.com) between June 13, 2016 and June 24, 2016. The survey was developed by the investigators in consultation with core members of the Canadian Vasculitis Network (CanVasc). The survey was designed to assess how Canadian rheumatologists define, diagnose, monitor, and treat patients with IA and IsA. A copy of the survey is available by clicking here. Prior to dissemination, the survey was piloted with a small group (n=4) of rheumatologists at our institution; they provided additional comments and approved the final version.
An e-mail invitation with a link to the survey was sent to members of the CRA by the CRA Communications branch; the survey was offered in English and French. Participants’ completion of the online survey constituted implied consent; participation was anonymous. Participants had two weeks to complete the survey; two reminder emails were sent out, at day 7 and on the last day of the survey.
Survey analysis
Data was extracted by the FluidSurveys™ software, and Microsoft Excel software (version 2010) was used for descriptive analysis. Ethics approval was obtained through the Ottawa Hospital Research Institute [(OHRI-RED protocol #4473)].
Results
Seventy-four of the 420 (18%) members of the Canadian Rheumatology Association responded, 68 (16%) took the survey, and 60/68 (88%) completed it.
Demographics
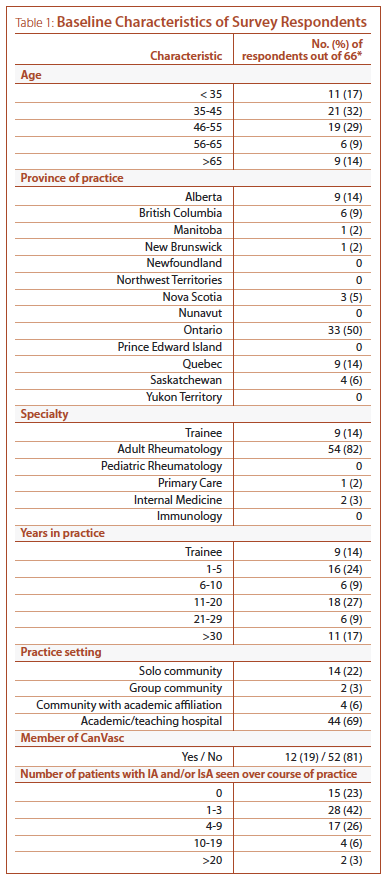
Baseline characteristics of respondents are presented in Table 1. The majority of participants were adult rheumatologists (54/66, 82%) between ages 35 and 55 (40/66, 61%), practicing at an academic institution (44/64, 69%), with half (33/66) of respondents based in Ontario. Twelve participants (19%) were core or associate members of CanVasc. Fifteen of the 66 (23%) participants reported having never seen a patient with IA or IsA over the course of their practice (Table 1); these subjects did not proceed to subsequent parts of the survey.
Definitions
Nearly all participants (46/47) felt excluding a defined systemic inflammatory condition was required for definition of IA. In addition, nearly half of participants (20/44) felt exclusion of radiographic abnormalities in aortic branch vessels was also required. The majority of participants (39/45, 87%) reported that inflammatory markers were irrelevant when diagnosing idiopathic aortitis.
Twelve of 47 participants (26%) reported making a distinction between IA and IsA. Of these participants, most considered exclusion of a defined systemic inflammatory condition (10/12, 83%) and radiographic abnormalities in aortic branch vessels (9/12, 75%) were required for the definition of IsA, and 9/12 (75%) felt that inflammatory markers were irrelevant for this definition.
Referrals
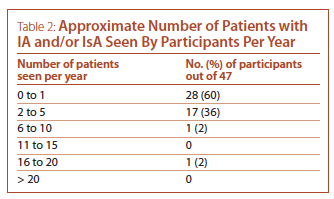
Vascular or cardiac surgery were the most common sources of referrals of patients with IA and IsA, having referred patients to 40/46 (87%) of participants. The majority of participants see one or fewer new patients with IA and/or IsA per year (see Table 2). The most common reason for referrals were the incidental finding of a vascular abnormality suggestive of aortitis on an imaging study (39/47, 83%) and the finding of positive pathology for aortitis post-aneurysm or aortic valve repair (34/47, 72%). Less common reasons for referrals were discovery of a thoracic aortic aneurysm in a patient with systemic symptoms/signs or other features of a systemic inflammatory condition (23/47, 49%), and discovery of a thoracic aortic aneurysm in a patient with past history of a defined systemic inflammatory condition known to be associated with aortitis (13/47, 28%).
Initial workup
Forty-five participants answered questions regarding initial workup of patients with IA; 13/45 (29%) reported they were the referral expert physician for vasculitis at their center (8 were CanVasc members). When assessing patients with suspected IA or IsA, most participants “always” screened for symptoms or signs suggestive of aortic branch vessel involvement (41/45, 91%), symptoms or signs of a defined systemic inflammatory condition (41/45, 91%), and for infectious signs and symptoms (38/45, 84%). With regards to laboratory investigations, all 45 respondents reported regularly testing complete blood count (CBC), renal function, and C-reactive protein (CRP). However, consistent testing to exclude tuberculosis and syphilis is less common, reported by 19 (42%) and 35 (78%) participants, respectively. Performing consistent cross-sectional imaging (CT or MR) of the whole aortic tree and its major branches in chest and abdomen was reported by 17 (38%) participants.
Treatment
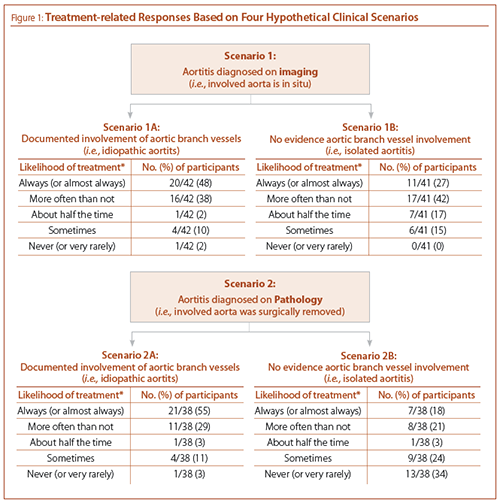
Participants were asked to indicate their treatment approach to four hypothetical clinical scenarios (see Figure 1): aortitis diagnosed on imaging with and without aortic branch vessel involvement (scenarios 1A and 1B, respectively), and aortitis diagnosed on pathology (with the involved area of aorta surgically removed) with and without aortic branch vessel involvement (scenarios 2A and 2B, respectively). Irrespective of the mode of diagnosis, participants were more likely to treat (with corticosteroids) aortitis with aortic branch vessel involvement. Participants were least likely to treat isolated aortitis with the involved aorta surgically removed (scenario 2B), with more than a third of participants “never” treating such patients. Notably, we did not find significant differences in treatment approaches of participants by type of practice, including practicing at a vasculitis referral center.
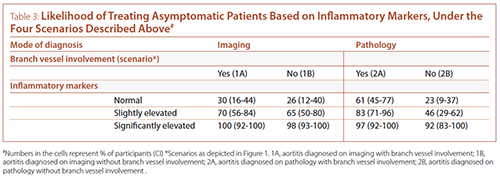
For each of the clinical scenarios, participants were then asked whether they would treat (with corticosteroids) asymptomatic patients in the setting of different levels of systemic inflammatory response (as assessed by inflammatory markers, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP)) (see Table 3). Most participants reported they would treat asymptomatic patients with significantly elevated inflammatory markers irrespective of the clinical scenario. In the setting of normal or mildly elevated inflammatory markers, participants were most likely to treat aortitis diagnosed on pathology with presence of additional aortic and/or branch vessel lesions (scenario 2A).
Follow up and monitoring
Most respondents reassessed their patients with IA every three months in the first two years after diagnosis (27/38, 71%). More than three quarters of respondents were following these patients with CBC, creatinine, ESR, and CRP at every visit. In addition, the majority of participants were performing CT (angiogram) or MR (angiogram) every 6 to 12 months (28/35, 80%). Notably, 4 respondents (11%) reported never ordering follow-up imaging for IA patients with documented radiographic involvement of aortic branch vessels at baseline. The monitoring approaches did not differ for aortitis diagnosed on imaging or on pathology.
When asked about participants’ comfort level in managing patients with IA or IsA, the majority of participants responded being “somewhat uncomfortable” 17/38 (45%), followed by “reasonably comfortable” 14/38 (37%), and “very uncomfortable” 5/38 (13%); two participants (5%) reported feeling “perfectly comfortable” managing these patients. Thirty six of 37 participants (97%) felt that the development of recommendations for the management of patients with IA and/or IsA would be beneficial.
Interpretation
Canadian rheumatologists are not familiar with IA and IsA, with nearly a quarter of participants reporting having never seen a patient with these conditions in their practice. The majority of participants reported seeing one or fewer cases per year. Only a small percentage of participants (5%) reported being “perfectly comfortable” managing patients with IA and/or IsA. As a result of insufficient volume of IA patients combined with lack of clinical guidelines, great variability was observed in this study with respect to various aspects of management of IA.
Only a quarter of participants reported making a distinction between IA and IsA. This is not surprising, given that the two terms are frequently used interchangeably in published literature.1-2,4,9 We consider IA when aortitis is seen in the absence of clinical features sufficient for diagnosis of an underlying systemic condition, most commonly GCA. IsA is a specific subtype of IA that is confined to the aorta. Complete imaging of the aortic branch vessels would be required to exclude branch vessel involvement and allow the diagnosis of IsA; reassuringly, three quarters of participants who made the distinction between IA and IsA considered exclusion of radiographic abnormalities in aortic branch vessels important for definition of IsA. Although the Chapel Hill nomenclature’s classification of IsA as a “single organ vasculitis” suggests that significant level of systemic inflammation should not be seen in this condition, this is not the case in our experience,6 the experience of participants of this study (75% of whom felt the level of inflammatory markers was irrelevant for definition of IsA), and in published literature.5
The majority of respondents performed thorough clinical and biochemical assessments of patients with IA and IsA. However, only 38% performed full imaging of chest and abdominal aortic branch vessels. In the case series of IA from the Mayo Clinic,2 the majority of patients (89%) underwent additional vascular imaging (i.e., CT and MR angiography). Additional vascular abnormalities were frequent, present in 72% of imaged patients. In the recently published case series from our centre, 21 of the 32 patients (66%) identified as having IA had complete imaging of branch vessels at baseline6; 15 (71%) of them were found to have branch vessel lesions and three (14%) had additional aortic lesions. In our opinion, given the high prevalence of additional vascular lesions, imaging of the whole aortic tree and its branches should be a standard part of the initial workup.
There is currently no standardized approach to medical therapy following diagnosis of IA, resulting in great uncertainty. The reported rates of corticosteroid use for treatment of IA range from 9% to 38% in the published literature2-4,6-7. Furthermore, there is a lack of information on how to direct treatment in specific clinical scenarios, such as presence of branch vessel disease or based on the level of inflammatory response. As would be expected, Canadian rheumatologists are more likely to treat disease with more extensive radiographic involvement or high levels of systemic inflammation. Interestingly, when faced with an asymptomatic patient with normal inflammatory markers, our participants appear to be most likely to treat in the presence of branch vessel abnormalities, and with a histologic as opposed to radiographic diagnosis of aortitis. This likely points to the relatively bigger consensus on histologic definition of aortitis9 compared to radiographic definition, with the latter being an area of significant controversy.10
A significant weakness of our study is the low response rate of 18%. According to the CRA, a typical response rate of surveys of this nature is 20-30%. We suspect this lower than average response rate reflects the rarity of idiopathic aortitis, resulting in many CRA members not participating due to lack of applicability of the survey subject to their individual practice. Supporting this theory is the significant over-representation in the respondents of academic rheumatologists with a primary practice based at a teaching hospital (69% of all participants, 55% excluding trainees), where patients with rare diseases like IA are most likely to be referred; the percentage of all Canadian rheumatologists with a university-based practice was estimated to be 40% in a recently published national survey.11 Targeting CRA members likely contributed to the overrepresentation of academic rheumatologists in our study, as they are more likely to be members of the CRA than those in solo community practice.11 Further selection bias was likely introduced by increased likelihood of response from rheumatologists who personally know this study’s investigators; this is demonstrated by the overrepresentation of Ontario rheumatologists in this study (50%) compared to national estimates of 38%.11 The low response rate and the overrepresentation of academic rheumatologists limits the generalizability of our findings to the entire Canadian rheumatology community. However, our results represent the views of the group of rheumatologists who have the most experience in IA, and whose opinions will therefore be most valuable for shaping of future recommendations to guide management of these conditions. Researcher bias in the development of the survey is another potential weakness of this study. As the investigators of the study, we designed the survey based on our personal experiences and knowledge regarding aortitis. The specific questions and the proposed response options likely biased the participants’ answers towards our (investigators’) views. In an attempt to minimize such bias, the survey was reviewed and modified by the core members of the CanVasc society and piloted with a small group of rheumatologists prior to its dissemination.
In conclusion, great variability is observed amongst Canadian rheumatologists with respect to definitions, work-up, treatment, and monitoring of patients with IA and IsA. Members of the CRA report uncertainty when managing these patients, identifying a strong need for recommendations to guide decisions. Based on our literature review, this study is the first report to evaluate the practice patterns of Canadian rheumatologists (or any group of rheumatologists, as no similar studies in IA have been published) with regards to idiopathic arthritis. Additional high quality (more systematic and/or prospective) research should be the first step to clarifying the best approach to IA, which will ultimately allow development of these much-needed guidelines.
References:
1. Gornik HL, Creager MA. Aortitis. Circulation 2008; 117:3039-51.
2. Liang KP, Chowdhary VR, Michet CJ, et al. Noninfectious ascending aortitis: a case series of 64 patients. J Rheumatol 2009; 36:2290-7.
3. Rojo-Leyva F, Ratliff NB, Cosgrove DM 3rd, Hoffman GS. Study of 52 patients with idiopathic aortitis from a cohort of 1,204 surgical cases. Arthritis Rheum 2000; 43:901-7.
4. Miller DV, Isotalo PA, Weyand CM, et al. Surgical pathology of noninfectious ascending aortitis: a study of 45 cases with emphasis on an isolated variant. Am J Surg Path 2006; 30:1150-8.
5. Merkel PA. Noninfectious ascending aortitis: staying ahead of the curve. J Rheumatol 2009; 36:2137-40.
6. Murzin DL, Belanger EC, Veinot JP, Milman N. A case series of surgically diagnosed idiopathic aortitis in a Canadian centre: a retrospective study. CMAJ 2017; 5:483-7.
7. Clifford A, Arafat A, Idrees J, et al. Aortitis: Outcomes from a cohort of 196 patients [abstract]. American College of Rheumatology Annual Meeting. Boston. Arthritis Rheum 2014; 66:S1216-7.
8. Jennette JC, Falk RJ, Bacon PA, et al. 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013; 65:1-11.
9. Stone JR, Bruneval P, Angelini A, et al. Consensus statement on surgical pathology of the aorta from the Society of Cardiovascular Pathology and the Association for European Cardiovascular Pathology: 1. Inflammatory diseases. Cardiovasc Pathol 2015; 24:267-78.
10. Cinar I, Wang H, Stone JR. Clinically isolated aortitis: pitfalls, progress and possibilities. Cardiovasc Pathol 2017; 29:23-32.
11. Barber CE, Jewett L, Badley EM, et al. Stand up and be counted: measuring and mapping the rheumatology workforce in Canada. J Rheumatol 2017; 44:248-57.
Marissa Keenan, MD, MSc
Rheumatology Fellow,
Department of Rheumatology,
The Ottawa Hosptial
Ottawa, Ontario
Nataliya Milman, MD, FRCPC, MSc
Rheumatologist,
Department of Rheumatology,
The Ottawa Hosptial
Ottawa, Ontario
The Canadian Vasculitis Network (CanVasc)
|




