Summer 2017 (Volume 27, Number 2)
Top Ten Things Rheumatologists Should (And Might Not) Know
About the NIHB Program
By Cheryl Barnabe, MD, FRCPC, MSc;
Karen Fortin, BScPhm, ACPR;
Susan Pierce, BScPharm, ACPR;
Henry Averns, MB, ChB, FRCP (UK), FRCPC;
and David Robinson, MD, MSc, FRCPC
Download PDF
The CRA, through the Optimal Care Committee (formerly Access to Care Committee), maintains a relationship with the First Nations and Inuit Health Branch of Health Canada to ensure that rheumatology patients covered under the Non-Insured Health Benefits (NIHB) Program have equitable access to necessary therapy. This Top Ten will review aspects of coverage relevant to rheumatologists.
1. The NIHB Program provides benefits for registered Indians (recognized by Indian and Northern Affairs Canada) or an Inuk recognized by Nunavut Tunngavik Incorporated, Inuvialuit Regional Corporation or Makivik Corporation. The NIHB Program does not cover benefits for First Nations without Treaty Status, nor Métis patients. As the payer of last resort, any coverage patients may have through private plans or provincial public agencies should be accessed first.
2. There are five types of benefits addressed by the NIHB Program: i) pharmacy benefits; ii) medical transportation; iii) vision care; iv) dental care; and v) medical supplies and equipment.
3. NIHB has recently joined the Pan-Canadian Pharmaceutical Alliance (PCPA) which will guide future formulary listings. For pharmacy benefits, the NIHB Formulary is published annually, and quarterly updates are provided. The available agents are listed at the following website:https://www.canada.ca/en/health-canada/services/first-nations-inuit-health/non-insured-health-benefits/health-provider-information/drug-pharmacy-information/drug-benefit-list-health-provider-drug-pharmacy-information-non-insured-health-benefits-first-nations-inuit-health-canada.html. The Optimal Care Committee maintains a regularly updated file of access criteria and response criteria on the CRA website: https://rheum.ca/en/members/non_insured_health_benefits_nihb. The other benefit areas are also found on the NIHB website:
https://www.canada.ca/en/health-canada/services/non-insured-health-benefits-first-nations-inuit.html
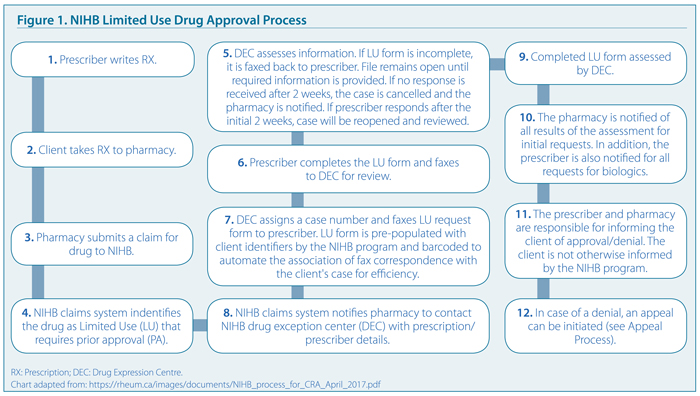
4. Disease-modifying antirheumatic drugs (DMARDs, including pre-filled methotrexate syringes), corticosteroids, nonsteroidal anti-inflammatory drugs (NSAIDs) and allopurinol are open benefits, do not require prior approval, and can be dispensed at the pharmacy visit. For Limited Use agents such as biologics and targeted synthetic DMARDs, a prior approval process is in place. Once the prescription is submitted to the pharmacy, a claim is initiated and this will generate the forms for completion that are faxed to the prescriber/physician. Once the forms are returned by the prescriber, the information provided on the forms is reviewed by a claims specialist, a dispensing history is checked, and a decision is made on whether the request is approved or denied. Figure 1 provides an overview of this approval process.
5. For access to Limited Use agents in rheumatology indications, the prescriber must be a rheumatologist. If the Limited Use criteria are met, approval for at least a one-year period is provided. The NIHB Formulary and the CRA “cheat sheet” contain the most updated information on initial access criteria, but Table 1 provides a general overview of the Limited Use criteria for different indications.
6. For renewal of Limited Use criteria, a new prescription is required, which will reinitiate the approval process. The NIHB Formulary and the CRA “cheat sheet” contain the most updated information on renewal criteria, but Table 2 provides a general overview of the Limited Use criteria for different indications. A one-year renewal period is provided, but for patients with a continuous demonstrated response to an agent, approval up to five years will be provided depending on the indication being treated.
7. An appeal process can be initiated when coverage for a benefit is denied. Appeals are submitted by the client, parent/legal guardian or representative of the client.Thus, some rheumatologists obtain written consent at the initiation of therapy to act as a representative. At the first level of appeal, a written and signed letter is submitted by mail indicating the client information (Indian/Inuit registration number and date of birth); name and address of the prescriber; the pharmacy where the medication was denied; the condition for which the medication is being requested; the diagnosis, prognosis and alternatives tried; relevant diagnostic test results, and other supporting information such as case notes. Level 1 appeals are reviewed by the manager of the Pharmacy Policy Development Division; Level 2 appeals are reviewed by the Director for the Benefit Management and Review Services Division; and Level 3 appeals by the NIHB Director General. Details on the appeal process are found at: http://healthycanadians.gc.ca/health-system-systeme-sante/services/non-insured-health-benefits-services-sante-non-assures/appealing-decision-faire-appel/index-eng.php. Appeals for vision care, medical transportation, medical supplies and equipment and mental health counselling are submitted to the NIHB Program in the province or territory of residence.
8. Coverage for osteoarthritis management includes intra-articular steroid preparations. Intra-articular hyaluronic acid preparations are only approved as an Exception for osteoarthritis of the knee when other treatments have failed. Topical NSAIDs (Pennsaid 1.5% and compounded) are covered but a Limited Use form will need to be completed. Assistive devices (canes, braces, etc.) are provided on a limited provision basis, and providers must submit claims and require receive approval for the devices before they are dispensed.
9. Recently, an opioid equivalent limitation has been set in place in the program. Oral adjunctive pain treatments that are open benefit include serotonin-specific reuptake inhibitors (SSRIs), gabapentin, duloxetine, tricyclic antidepressants and anticonvulsants, such as valproate and topiramate. Pregabalin and nabilone are Limited Use therapies.
10. If you are having difficulty with any aspect of NIHB Program coverage, the number for the Drug Exception Centre is 1-800-580-0950 (Press 2 to speak with a supervisor). Patients may also contact their NIHB Navigator to assist with the process.
Cheryl Barnabe, MD, FRCPC, MSc
Associate Professor,
University of Calgary
Calgary, Alberta
On behalf of Karen Fortin, BScPhm, ACPR;
Susan Pierce,
BScPharm, ACPR;
Henry Averns, MB ChB, FRCP (UK), FRCPC;
and David Robinson, MD, MSc, FRCPC
|




