Fall 2016 (Volume 26, Number 3)
CRA Acetaminophen Survey Results
Download PDF
Discovered more than a century ago, acetaminophen has become one of the most widely used over-the-counter (OTC) compounds for pain and fever relief. Due to its safety and effectiveness, not surprisingly, the World Health Organization (WHO) considers it to be one of its essential medicines.1
Reports of overdoses and adverse effects, particularly on the liver, however, have brought forth questions as to whether Canadian regulations surrounding acetaminophen should be altered.2 Some studies indicate that acetaminophen may not be as safe as previously thought.3 We asked CRA members for their opinions and perspectives on acetaminophen and whether they see the need for changes to its accessibility.
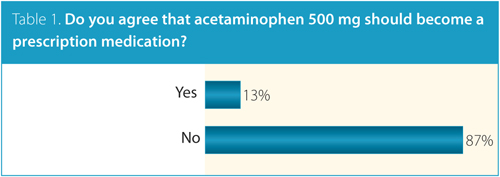
Currently in Canada, acetaminophen is available OTC in unit doses ranging from 80 mg to 650 mg. When asked whether acetaminophen 500 mg should become a prescription medication, most survey respondents, almost 87%, agreed that it should not (Table 1). Some of you explained your opposition to restricting access, citing practical concerns over placing undue strain on the healthcare system. One CRA member explained, “If acetaminophen 500 mg becomes a prescription drug, there will be too much extra work for GPs to handle the needed appointments for prescription requests and renewals. Let the pharmacists regulate this, they are well-trained.” Several other comments echoed similar sentiments. As well, there were concerns that making acetaminophen harder to obtain would lead patients to substitute OTC nonsteroidal anti-inflammatory drugs (NSAIDs), leading to more organ-specific toxicities. “Do not restrict acetaminophen,” wrote one CRA member, “as it will force patients to take more over-the-counter NSAIDS and this will have far more negative effects with an increase of GI hemorrhage events, renal failure and most probably coronary events too!”
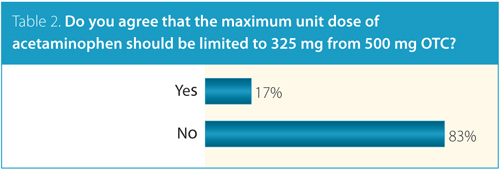
When asked about reducing the maximum unit dose OTC to 325 mg, the vast majority of CRA members (83%) also agreed that this should not be done (Table 2).
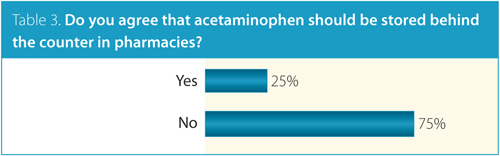
Three quarters of respondents also opposed placing acetaminophen behind the counter in pharmacies (Table 3). One survey respondent wrote, “As long as companies marketing acetaminophen ensure clear labelling, I don’t see the need to restrict access to this antipyretic and analgesic medication. Perhaps, reducing the number of pills per container or packaging as blister packs would decrease the risks of unintentional overdoses.”
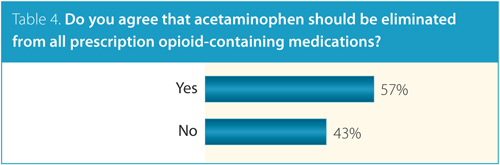
Of all the survey questions, the most polarizing was whether acetaminophen should be included in opioid-containing medications. More than half of respondents (57%) supported the elimination of acetaminophen from all prescription opioid-containing medications (Table 4).
Overall, while most CRA members agreed with maintaining the current standards for OTC acetaminophen, there seemed to be some concern with regard to the safety of acetaminophen in prescription opioid-containing medications.
This Joint Count article was developed and supported by the CRA Therapeutics Committee to help guide a response to the Health Canada panel.
References:
1. World Health Organization. WHO Model Lists of Essential Medicines. http://www.who.int/medicines/publications/essentialmedicines/EML_2015_FINAL_amended_NOV2015.pdf?ua=1. Published April 2015. Accessed July 28, 2016.
2. Health Canada. Summer Safety Review – Acetaminophen – Liver Injury. http://www.hc-sc.gc.ca/dhp-mps/medeff/reviews-examens/acetamino-eng.php. Published July 9, 2015. Accessed July 28, 2016.
3. Brune K, Renner B, Tiegs G. Acetaminophen/paracetamol: A history of errors, failures, and false decisions. Eur J Pain. 2015 Aug;19 (7):953-65.
|