Summer 2014 (Volume 24, Number 2)
RA Guidelines: Practice
Practice Patterns of Rheumatologists in Canada Compared to the CRA Recommendations for RA
(Part V)
By Sankalp V. Bhavsar, MD, FRCPC;
on behalf of Carter Thorne, MD, FRCPC, FACP;
Claire Bombardier, MD, FRCPC;
Vivian P. Bykerk, MD, FRCPC;
Glen S. Hazlewood, MD, FRCPC;
Pooneh Akhavan, MD, FRCPC;
Orit Schieir, MSc; and
Sanjay Dixit, MD, FRCPC
Download PDF
In this installment, we present the results of survey questions pertaining to peri-operative care, treatment of latent tuberculosis infection, and vaccinations
1. A patient with rheumatoid arthritis (RA) is maintained on adalimumab and methotrexate (MTX). She is scheduled for elective cholecystectomy. Regarding peri-operative management of adalimumab, you would suggest:
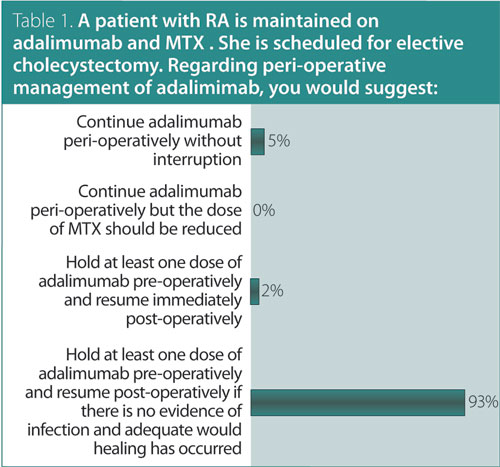
Answer: Hold at least one dose of adalimumab pre-operatively and resume post-operatively if there is no evidence of infection and adequate wound healing has occurred.
Recommendation/supporting evidence: American College of Rheumatology (ACR) 2008,1 Spanish Society of Rheumatology (SER) 2010.2
Both ACR 20081 and SER 20102 guidelines referred to the same three cohort studies that examined risks for postoperative infections in RA patients treated with anti-tumor necrosis factor (TNF) agents. The largest retrospective cohort study of 768 RA patients underwent 1,219 elective orthopedic procedures and reported nonsignificant increased odds ratios (OR) for surgical site infections in patients who continued anti-TNF therapy (OR 1.5, 95% CI 0.43–5.2). In that study, surgical site infections were observed in 41/1,023 (4%) patients who did not use anti-TNF, 6/104 (5.8%) patients that stopped anti-TNF therapy (for > 4 half-lives), and 8/92 (8.7%) patients that continued anti-TNF therapy. A retrospective cohort study of 91 RA patients who underwent orthopedic surgery reported a higher incidence of peri-operative infections in patients treated with anti-TNF therapy relative to patients who were not treated with an anti-TNF (OR 5.3, 95% CI 1.1–24.9). A small prospective cohort of 31 RA patients who underwent orthopedic surgery did not report a significant increase in post-operative infections or healing complications associated with anti-TNF therapy.1,2
2. A patient with RA has a positive tuberculin skin test (induration of
12 mm). Regarding initiation of anti-TNF therapy and latent tuberculosis infection (isoniazid), you would suggest:
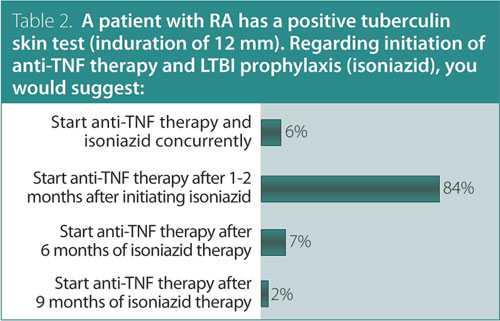
Answer: Start anti-TNF therapy one to two months after initiating isoniazid.
Recommendation/supporting evidence: Furst 2010.3
Furst3 reviewed observational evidence from Spain, which showed that RA patients who screened positive for latent tuberculosis infection (LTBI) and were treated with anti-TNF therapy following one month of tuberculosis (TB) prophylaxis had a significantly reduced risk of TB reactivation.
3. Which of the following statements regarding vaccinations in RA is false?
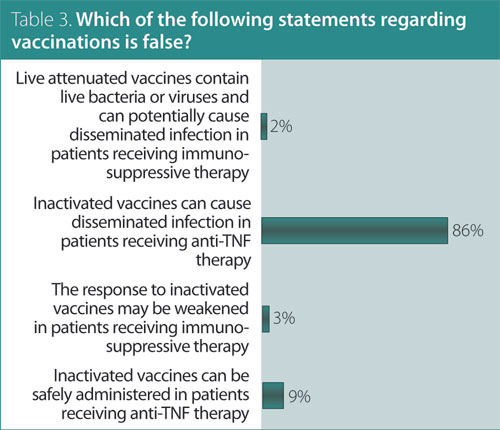
Answer: Herpes zoster (HZ) vaccine can be safely administered in patients 60 years or older receiving anti-TNF therapy.
Recommendation/supporting evidence: European League Against Rheumatism (EULAR) 2011,4 the Canadian Immunization Guide 2006,5 and US Centers for Disease Control and Prevention (CDC) 2011.6
Evidence for the recommendation was based on results of a recent systematic review undertaken to inform EULAR 2011 recommendations for patients with autoimmune inflammatory rheumatic diseases.4
Influenza. One observational study showed a reduction in infections at one year in 34 patients with RA who received influenza vaccine, compared to 20 patients who did not (instances of acute bronchitis were 22.6% vs. 4.3%, respectively; viral respiratory infections 61.3% vs. 8.7%). Two other observational studies also found a reduction in hospital admissions and mortality from influenza and pneumonia in elderly patients with rheumatic diseases who received the influenza vaccine.
HZ. An increased risk of HZ has been reported in RA patients compared to healthy population controls from two administrative databases (adjusted hazard rate ratios 1.7 and 1.9, respectively). In addition, treatment with glucocorticoids, azathioprine, leflunomide, and anti-TNF was associated with increased risks of HZ.
In general, immunocompromised persons should not receive live vaccines because of the risk of disease caused by the vaccine strain. However, literature suggests that HZ vaccine can be safely administered to patients on low-dose immunosuppression, and it is reasonable to consider HZ vaccine in individuals receiving such therapy
(e.g., MTX ≤ 0.4 mg/kg/week). The 2014 publication of the Canadian Immunization Guide5 states that it is reasonable to consider HZ vaccine in patients receiving anti-TNF biologics on a case-by-case basis after review with an expert in immunodeficiency. Retrospective data demonstrates the safety of HZ vaccine in people receiving anti-TNF therapy for inflammatory conditions. However, the current CRA recommendation is to avoid HZ vaccine in patients receiving biologic therapy.6
For further information on these recommendations and the supporting evidence of these results, please consult the CRA RA Guidelines document, available at www.rheum.ca/en/
publications/cra_ra_guidelines.
References
1. Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis [review]. Arthritis Rheum 2008; 59(6):762-84.
2. Spanish Society of Rheumatology. Update of the Consensus Statement of the Spanish Society of Rheumatology on the management of biologic therapies in rheumatoid arthritis. Rheumatol Clin 2010; 6(1):23-36.
3. Furst DE, Keystone EC, Fleischmann R, et al. Updated consensus statement on biological agents for the treatment of rheumatic diseases 2009. Ann Rheum Dis 2010;69 Suppl 1:i2-29.
4. van Assen S, Agmon-Levin N, Elkayam O, et al. EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis 2011; 70(3):414-22.
5. National Advisory Committee on Immunization. Canadian Immunization Guide: Public Health Agency of Canada; 2006 and 2014. Available at www.phac-aspc.gc.ca/publicat/cig-gci/.
6. Centers for Disease Control and Prevention. General recommendations on immunization - Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60(2):1-64.
Sankalp V. Bhavsar, MD, FRCPC
Rheumatology Fellow,
McMaster University
Hamilton, Ontario
on behalf of Carter Thorne, MD, FRCPC, FACP;
Claire Bombardier, MD, FRCPC;
Vivian P. Bykerk, MD, FRCPC;
Glen S. Hazlewood, MD, FRCPC;
Pooneh Akhavan, MD, FRCPC;
Orit Schieir, MSc; and
Sanjay Dixit, MD, FRCPC |



