Spring 2014 (Volume 24, Number 1)
Update on Immunization in Patients with Autoimmune Rheumatic Diseases
By Shelly McNeil, MD, FRCPC; and Diva Miri, MD
Download PDF
Mrs. M is a 56-year-old woman with longstanding rheumatoid arthritis (RA) for which she has been maintained on methotrexate (MTX) for many years. Because of ongoing disease activity and functional limitation, hydroxychloroquine was added three months ago. Despite this, she has had ongoing disease activity and a decision has been made to initiate therapy with infliximab. Prior to starting this agent, you wish to review her immunization history and ensure that she has been brought up to date on all recommended vaccinations.
Patients with chronic inflammatory rheumatic or autoimmune conditions are known to be at approximately two-fold greater risk of severe infections than healthy adults. While the mechanism of increased risk is not entirely understood, it is likely to be multifactorial and involve aberrations in immune system function, prevalent underlying comorbidity, and the immunosuppressive properties of many of the therapeutic agents used to manage disease. Immunization therefore represents an important and sometimes overlooked element of the care of these patients.
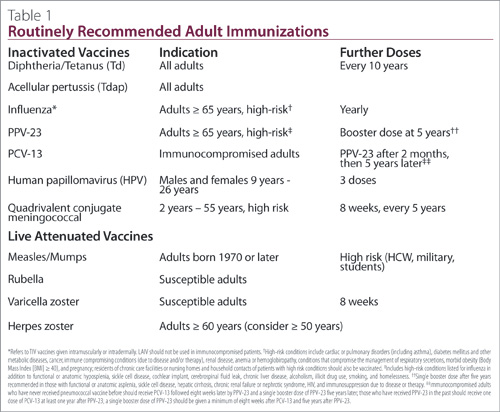
Several principals guide the immunization of immunocompromised patients due to disease or therapy. Patients with underlying autoimmune rheumatic diseases (RD) and those undergoing treatment with immunosuppressive medications should receive all routinely recommended inactivated adult vaccines (Table 1). In general, patients receiving immunosuppressive doses of medications should not receive live attenuated vaccines because of the risk of disseminated disease caused by the vaccine strains; the decision to withhold live vaccines should be made after careful assessment of the risk:benefit of a particular live vaccine in an individual patient given the patient’s underlying diagnosis and degree of immunosuppression. Many patients with chronic inflammatory conditions are cared for by both primary-care and specialty physicians, so it is critical that rheumatologists actively review immunization histories at each visit and seize opportunities to provide all recommended vaccines, or provide clear guidance to primary-care physicians about which vaccines should be provided or avoided. Because the immune response to some vaccines may be suboptimal in this population, protection should be optimized by providing recommended vaccines as early in the disease course as possible and, ideally, before initiation of immunosuppressive medications. Protection should also be optimized by ensuring that all household and other close contacts of these patients have received all recommended vaccines.
Further history from Mrs. M reveals that she has received all recommended childhood vaccines, but has never received any vaccines as an adult. She works as a librarian, has three adult children and one infant grandchild. She is married and monogamous. Aside from her RA, she is well and takes no medications other than her MTX and hydroxychloroquine.
Like all adults, Mrs. M should receive a single dose of tetanus-diphtheria-acellular pertussis (Tdap) vaccine. While serious disease due to pertussis typically occurs in young infants, approximately 20% of all cough illness in adults lasting longer than 10 days is due to pertussis. The incidence of pertussis is increasing in adolescents and adults since the advent of routine pertussis immunization in childhood. Administration of Tdap is of particular importance in Mrs. M because of her risk of transmitting pertussis to her infant grandchild, who would be at high risk of severe disease. Following a single dose of Tdap, Mrs. M should continue to receive a booster dose of tetanus-diphtheria toxoid (Td) every 10 years.
Annual influenza immunization is recommended in patients immunocompromised due to disease or therapy. Only trivalent inactivated influenza vaccine (TIV) should be used in this population. Live attenuated influenza vaccine (LAIV) should not be used, but is safe in household contacts of immunocompromised patients. Although influenza vaccine effectiveness may be lower than in healthy adults, limited studies suggest that the majority of immunocompromised adults will mount a protective humoral antibody response to TIV. Delivery of TIV into the dermis of the skin has the potential to enhance the immune response to influenza vaccine by exposing the vaccine antigen to antigen-presenting dendritic cells present in high concentrations in the dermis. Amongst adults aged 60 years and older, intradermal TIV has been shown to elicit immune responses that are superior to conventional intramuscular TIV. Based upon improved immunogenicity in the elderly, it is reasonable to consider the use of intradermal TIV in younger immunocompromised patients to optimize protection. A high-dose TIV vaccine containing four times the influenza antigen present in conventional TIV products has been shown to elicit higher immune responses in elderly adults and in patients with human immunodeficiency virus. When this vaccine becomes available in Canada in the near future, it will offer an alternative with improved immunogenicity and potentially improved effectiveness in immunocompromised adults. Studies of the immunogenicity and efficacy of adjuvanted TIV, intradermal TIV, quadrivalent and high-dose TIV are urgently needed to inform decisions about the preferred influenza vaccination strategy in this high-risk population.
Recommendations for the prevention of pneumococcal disease in immunocompromised adults have recently changed in Canada (Table 1). Invasive pneumococcal disease is an important cause of morbidity and mortality in immunocompromised adults, those 65 years and older and adults of all ages with medical comorbidities. There are currently two vaccines available for the prevention of pneumococcal disease in adults. The immunogenicity of 23-valent pneumococcal polysaccharide vaccine (PPV-23) has been evaluated in patients with chronic rheumatic or autoimmune diseases with mixed results. In general, patients with systemic lupus erythematosus (SLE), RA, and those on disease-modifying antirheumatic drugs (DMARDs), including monotherapy with MTX, tend to mount protective immune responses less frequently than healthy adults to some vaccine strains. Some data suggest the duration of antibody protection may also be reduced. A 13-valent conjugated pneumococcal vaccine (PCV-13) has recently been authorized for use in adults in Canada and is now recommended by the National Advisory Committee on Immunization (NACI), for all immunocompromised adults. While no studies of vaccine effectiveness of PCV-13 have been done, PCV-7, the 7-valent pneumococcal conjugate vaccine used prior to
PCV-13 has been shown to prevent invasive pneumococcal disease in HIV-infected adults; PPV-23 was not effective in such patients. Studies of the immunogenicity of PCV-13 in immunocompromised adults have shown mixed results but, in general, PCV-13 appears to be more immunogenic in patients who have undergone hematopoietic stem cell transplant and those with HIV infection; the data is less convincing in patients with solid organ transplant. There have been no studies of immunogenicity or effectiveness of PCV-13 in patients with autoimmune RDs. Given the importance of invasive pneumococcal disease as a cause of morbidity and mortality in immunocompromised adults, the suboptimal effectiveness of PPV-23, and the potential immunologic advantages of PCV-13, NACI now recommends that all immunocompromised patients receive both PCV-13 and PPV-23 to provide optimal humoral immunity against the 13 pneumococcal strains in PCV-13, and to broaden coverage against the additional pneumococcal strains in PPV-23. Because the immune response to PCV-13 is impaired in patients who have recently received PPV-23, the timing of administration of these vaccines in very important. Patients who have not received PPV-23 should receive a dose of PCV-13 followed eight weeks later by a dose of PPV-23; a single booster dose of PPV-23 five years later completes the series. Patients who have had PPV-23 in the past should receive a dose of PCV-13 at least one year after the dose of PPV-23; a single booster dose of PPV-23 should be given at least eight weeks after the PCV-13 and five years after PPV-23 to complete the series.
Mrs. M should receive a dose of PCV-13 now followed by PPV-23 in eight weeks and a booster in five years. PCV-13 and PPV-23 can be co-administered with influenza vaccine for convenience.
Shingles, caused by reactivation of latent varicella zoster virus from the spinal and cranial sensory ganglia, is characterized typically by unilateral pain and vesicular rash in a dermatomal distribution. Approximately one in three adults will develop shingles in their lifetime; rates of shingles in immunocompromised adults are two- to five- fold higher than in the general population. Patients with RA have a rate of shingles of
13-14 cases per 1,000 person-years compared to 1.5-4 cases per 1,000 person-years in healthy adults. Additionally, persons with immunocompromise are much more likely to experience complications of shingles, including the risk of disseminated disease and much higher rates of post-herpetic neuralgia, a chronic, debilitating neuropathic pain syndrome. Thus, prevention of shingles is a high-priority area of vaccine research and development.
At present, there is only one licensed shingles vaccine, a live-attenuated herpes zoster vaccine which, in clinical trials, has been shown to prevent approximately half of all shingles cases and two-thirds of cases of post-herpetic neuralgia in healthy adults aged 60 years and older and to have slightly better efficacy in adults 50 to 59 years old. The herpes zoster vaccine is currently recommended for all Canadian adults aged 60 years and older and may be considered in those aged 50 years and older who desire protection from shingles, or who are anticipating immunosuppression which would put them at increased risk of disease. In general, live-attenuated shingles vaccine is contraindicated in the immunocompromised. Chronic inflammatory, RD and autoimmune diseases are not, in themselves, a contraindication to this vaccine; however, many of the medications used to treat these conditions are sufficiently immunosuppressive to warrant caution, and there is little data to guide decision-making with some routinely used DMARDs. Given the disproportionate burden of shingles in the immunocompromised population, careful risk:benefit assessment should be undertaken in patients receiving or intending to start DMARDs. When possible, herpes zoster vaccine should be administered prior to initiation of immunosuppressive therapy to optimize immunogenicity and safety. Ideally, the vaccine should be administered two to four weeks prior to initiation of therapy. In patients already on immunosuppressive agents that contraindicate this vaccine, consideration should be given to withholding the immunosuppressive to provide opportunity to administer needed vaccines. The period between holding an agent and giving a live-attenuated vaccine must be determined based on the pharmacologic properties of the agent, but should generally not be less than one month.
The herpes zoster vaccine can safely be administered to patients on low-dose steroids (< 20mg/d of prednisone or its equivalent), MTX
(≤ 0.4mg/kg/week), azathioprine (≤ 3.0mg/kg/d) and 6-mercaptopurine
(≤ 1.5mg/kg/d). There is a limited but growing body of evidence that the vaccines may be safe and effective in patients on anti-tumor necrosis factor (TNF) biologic agents. In a study assessing safety of the herpes zoster vaccine in almost 45,000 patients with a wide array of RDs using linked datasets in the US, 47 patients who had received anti-TNF agents within 30 days before or after receipt of the vaccine were identified. None of them experienced serious adverse events and none developed shingles within 42 days of vaccination. In a similar study examining risk of zoster in almost 464,000 patients with underlying autoimmune disorders, 663 patients receiving anti-TNF agents at the time of vaccination were identified. Again, none experienced a serious adverse event and none developed zoster. In fact, the hazard ratio for herpes zoster associated with vaccination was 0.61 (95% CI 0.52, 0.71), suggesting a protective effect of vaccination even in patients receiving anti-TNF agents. Based on this data, the NACI now recommends that the herpes zoster vaccines may be administered to patients on anti-TNF biologics on a case by case basis after review with an expert in immunodeficiency. There is insufficient data to support this approach in patients on non-anti-TNF biologics or a combination of anti-TNF biologics and other immunosuppressive DMARDs.
New heat-inactivated and subunit herpes zoster vaccines are currently in Phase 2 and 3 clinical trials and may fill an important clinical gap in preventing shingles among the growing population of immunocompromised persons.
Given Mrs. M’s age and anticipated need for anti-TNF therapy, she should be offered live attenuated shingles vaccine at least two weeks, but ideally four weeks prior to starting infliximab. Mrs. M should be vaccinated even if she does not recall having chickenpox as a child as the vast majority of Canadian adults have been exposed to varicella zoster virus. No serologic testing is required before or after vaccination. Given the increased risk of recurrent zoster in immunocompromised adults, Mrs. M should be offered herpes zoster vaccine even if she has had a prior episode of shingles. In that setting, administration of the vaccine should be delayed at least one year following resolution of shingles, as it is likely that vaccine will not offer benefit over naturally augmented cell- mediated immunity in the year following a shingles episode. Live attenuated shingles vaccine can be administered on the same day as influenza vaccine, PPV-23, and PCV-13.
Suggested Readings
1. Bombardier C, Hazlewood GS, Pooneh A, et al. Canadian Rheumatology Association Recommendations for the Pharmacologic Management of Rheumatoid Arthritis with traditional and biologic disease-modifying antirheumatic drugs: Part II Safety. J Rheumatol 2012; 39(8):1583-602.
2. Public Health Agency of Canada. Canadian Immunization Guide. Available from: www.phac-aspc.gc.ca/publicat/cig-gci/index-eng.php
3. An Advisory Committee Statement (ACS) National Advisory Committee on Immunization: An addendum to the 2010-11 trivalent inactivated influenza vaccine: Recommendations on the use of intradermal trivalent inactivated influenza vaccine (TIV-ID). Available from: www.phac-aspc.gc.ca/publicat/ ccdr-rmtc/11vol37/acs-dcc-4/index-eng.php
4. McKittrick N, Frank I, Jacobson JM, White CJ, Kim D, Kappes R, et al. Improved immunogenicity with high dose seasonal influenza vaccine in HIV-infected persons. Ann Intern Med 2013; 158(1):19-26.
5. An Advisory Committee Statement (ACS) National Advisory Committee on Immunization: Statement on the use of conjugate pneumococcal vaccine-13 valent in adults (PNEU-C-13). Available from: www.phac-aspc.gc.ca/ publicat/ccdr-rmtc/13vol39/acs-dcc-5/assets/pdf/13vol39-acs-dcc5-eng.pdf
6. Public Health Agency of Canada. An Advisory Committee Statement (ACS) - National Advisory Committee on Immunization (NACI): Update on the Use of Herpes Zoster Vaccine. 2014. Available from: www.publications.gc.ca/ collections/collection_2014/aspc-phac/HP40-92-2014-eng.pdf
7. Gluck T, Muller-Ladner U. Vaccination in patients with chronic rheumatic or autoimmune diseases. Clin Infect Dis 2008; 46(9):1459-65.
Shelly McNeil, MD, FRCPC
Professor of Medicine,
Infectious Diseases
Canadian Center for Vaccinology,
IWK Health Centre
Halifax, Nova Scotia
Diva Miri, MD
Research Associate,
Canadian Center for Vaccinology,
IWK Health Centre and Capital Health,
Dalhousie University
Halifax, Nova Scotia |



