Spring 2014 (Volume 24, Number 1)
Rheumatoid Arthritis and Herpes Zoster
By Gordon Dow, MD, FRCPC
Download PDF
A 63-year old woman presented with a 12-year history of poorly controlled seropositive rheumatoid arthritis (RA) treated with oral methotrexate (MTX) 20 mg weekly and prednisone 7.5 mg-10 mg per day. While preparing to initiate anti-tumor necrosis factor (TNF) therapy, she developed exquisite pain over her left forehead. Analgesic therapy prescribed at a walk-in clinic was ineffective, and four days after onset of pain she developed erythema and multiple vesicles over the left side of the nose and left forehead. Twenty-four hours after onset of this rash, she presented to her rheumatologist for her next scheduled appointment.
At least 90% of adults in Canada have had previous infection with varicella-zoster virus (VZV) acquired in childhood, and are at increased risk for herpes zoster reactivation from latent reservoirs in cranial and dorsal root sensory ganglia. Adults have a 20%-30% lifetime risk of developing herpes zoster, with an incidence of approximately 130,000 cases in Canada each year.1 Risk increases with older age, local trauma, psychological stress, immunosuppressive conditions, and immunosuppressing medications. Herpes zoster is a significant concern for patients with RA and their caregivers because of the profound disability induced by both acute neuritis and postherpetic neuralgia (PHN). PHN is the most notorious adverse consequence associated with herpes zoster. It is usually defined as the persistence of pain more than four weeks after rash disappearance, but this definition is arbitrary and varies between studies. PHN is uncommon in persons younger than 60, and is not increased in the immunocompromised host. The incidence of PHN may be lower in persons receiving anti-TNF therapy.2
Are Patients with RA at Increased Risk for Herpes Zoster?
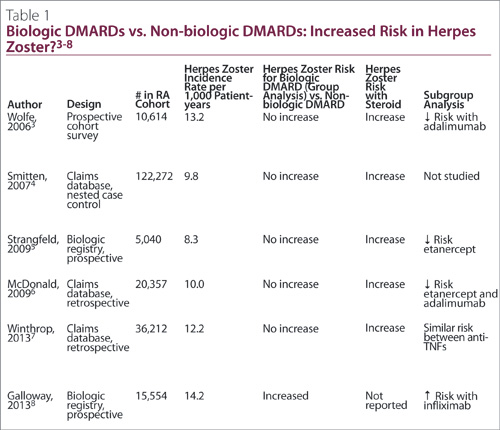
Several recent population-based studies3-8 of patients with RA have documented a crude incidence of herpes zoster of approximately
10/1,000 patient years. This is twice the risk documented in the non-RA population after adjusting for age.4 This increased risk is not merely due to immunosuppressive disease-modifying antirheumatic drug (DMARD) therapy. An incidence of 8.0 per 1,000 patient-years in RA patients on minimal therapy was documented, in contrast to occurrences of 11.2 and 10.6 per 1,000 patient-years for patients treated for moderate and severe disease, respectively.6 The risk of herpes zoster in the general population ranges from three to four per 1,000 patient-years.4,9 This data would suggest that the immune dysregulation of RA itself is associated with an increased risk of herpes zoster.
Which Immunosuppressive Medications Increase Patient Risk for Herpes Zoster?
Steroid therapy is unequivocally associated with herpes zoster reactivation, and its impact is dose-dependent. The impact of DMARD therapy is less clear, partly due to the confounding influence of concurrent steroid use in large cohort studies. A nested case control study4 found that oral steroids had the highest adjusted odds ratio (2.51), while the odds ratio for biologic DMARDs was similar to traditional DMARDs
(1.54 vs. 1.37). The risk for herpes zoster was similar regardless if steroid was used alone or combined with DMARD therapy. Significant increased herpes zoster risk has been documented with cyclophosphamide, azathioprine, prednisone, leflunomide, and COX-2 selective non-steroidal anti-inflammatory drugs (NSAIDs), but not with MTX or biologic DMARDs.3
It is currently unclear whether biologic DMARD therapy increases the risk of herpes zoster versus traditional DMARDs (Table 1). Only one study8 showed an increased risk with anti-TNF therapy when drugs in this class are evaluated in combination. This study8 had significant methodologic flaws, including use of patient self-report for case finding, and use of hospital-based clinics for the biologic DMARD cohort versus community-based clinics for the control population. Subgroup analyses in several studies have shown an increased risk for specific biologic DMARDs, particularly infliximab, as evinced in Table 1. The weight of evidence currently indicates that herpes zoster risk is similar between biologic and traditional DMARDs, and the more menacing culprit is corticosteroid therapy.
Should Patients with RA Be Vaccinated Against Herpes Zoster?
The effectiveness of herpes zoster vaccination in the patient with RA is unknown, given that immunocompromised persons were excluded from the two largest registration trials10,11 for this vaccine. In these two trials,10,11 vaccination decreased herpes zoster risk in healthy adults aged older than 60 by 51% (3.3% vs. 1.6%),10 and by 70% (0.88% vs. 0.27%) in adults aged 50 to 59.11 The Shingles Prevention Study (SPS)10 found the vaccine was well-tolerated, with mild inoculation-site side effects in 48% of vaccine recipients, versus 16% in the placebo group.
The SPS10 also documented a 67% reduction in PHN (0.1% vs. 0.4%). Based on this study,10 the number needed to vaccinate to prevent one case of shingles over three years was 59, and to prevent one case of PHN was 364. Longer-term follow-up of a patient subgroup from the SPS has shown that statistical significance for protection was lost by the third year post-vaccination for PHN and the sixth year post-vaccination for herpes zoster.12
A recent retrospective cohort study13 examined 463,541 Medicare beneficiaries with immune-mediated disease. It documented a 40% decrease in herpes zoster (HR 0.61; 95% CI 0.52-0.71) over two years, suggesting efficacy in the immunocompromised population.
Other impediments to vaccination include its cost (approximately $200), recent vaccine shortages, modest efficacy, and requirement for frozen storage and transport. Given that the risk of herpes zoster is two-to-three-fold higher in the RA population, carries significant risk of disability, and appears to be vaccine responsive, persons with RA age 50 or older can be offered vaccination after discussing the pros and cons above.
Should Patients Who Have Had a Previous Episode of Herpes Zoster Be Vaccinated?
Herpes zoster vaccine trials enrolled patients regardless of prior herpes zoster episodes. One recent study14 demonstrated that there is no added benefit to herpes zoster vaccination in persons with documented herpes zoster in the previous two years, suggesting that natural infection boosts cell-mediated immunity to VZV.
When Should Patients With RA Be Vaccinated Against Herpes Zoster?
Ideally, patients with RA should be offered herpes zoster vaccine more than two weeks prior to initiating immunosuppressive therapy. Live vaccines have traditionally been prohibited in patients receiving DMARD therapy or if receiving prednisone in doses greater or equal to 20 mg per day. Recent data has documented that vaccine risk with traditional DMARDs is low and vaccination in this subgroup is now permissible.15
A study13 identified 633 patients who received herpes zoster vaccination while receiving biologic therapy, with no evidence of herpes zoster or varicella in the ensuing 42 days. This finding provides only preliminary reassurance, however, given that claims databases are insensitive to vaccine complications. We have documented one patient with localized vaccine-induced herpes zoster while on infliximab therapy (material submitted for publication). VZV-associated retinal necrosis has been described in two immunocompromised patients after receiving herpes zoster vaccination.16 Herpes zoster vaccination cannot be presently recommended while patients are receiving biologic DMARD therapy. Holding this therapy for three half-lives would seem prudent based on current data, with resumption of biologic DMARD therapy two weeks
post-vaccination.
Return to Our Clinical Case
Early treatment of herpes zoster speeds healing and decreases the severity of acute neuritis but may have minimal impact on the risk of PHN. The greatest clinical benefit is derived if treatment is initiated within 72 hours after the onset of rash, especially in patients older than age 50, who are prone to more prolonged pain. Early initiation of therapy is even more important in the immunocompromised patient or any patient with herpes zoster ophthalmicus given their increased risk of complications. Antiviral therapy should be given to these patients even if they present after
72 hours.
The immunocompromised host with disseminated zoster or patient with sight-threatening disease should be hospitalized to receive intravenous acyclovir at a dose of 10 mg/kg three-times daily for seven days. Topical corticosteroid drops and ophthalmology assessment is required for patients with herpes zoster ophthalmicus. For patients who do not have herpes zoster ophthalmicus or other complications, we recommend valacyclovir 1,000 mg three-times daily or famciclovir 500 mg three-times daily for seven days. These medications are preferable to oral acyclovir, given its poor bioavailability and more frequent dosing requirement.
The best treatment for acute neuritis is rapid initiation of antiviral therapy and low dose amitriptyline (25 mg per day). There is no benefit to use of adjunctive corticosteroids or gabapentin.
Our patient made a complete recovery after being given valacyclovir
1,000 mg TID, amitriptyline 25 mg QHS, and topical ophthalmic steroid under the guidance of an ophthalmologist.
References
1. Brisson M, Pellissier JM, Camden S, et al. The potential cost-effectiveness of vaccination against herpes zoster and post-herpetic neuralgia. Hum Vaccin 2008; 4(3):238-45.
2. Javed S, Kamili QU, Mendosa N, et al. Possible association of lower rate of postherpetic neuralgia in patients on anti-tumor necrosis factor-alpha. J Med Virol 2011; 83(11):2051-5.
3. Wolfe F, Michaud K, Chakravarty EF. Rates and predictors of herpes zoster in patients with rheumatoid arthritis in non-inflammatory musculoskeletal disorders. Rheumatol 2006; 45(11):1370-5.
4. Smitten A, Choi H, Hochberg M, et al. The risk of herpes zoster in patients with rheumatoid arthritis in the United States and the United Kingdom. Arthritis Rheum 2007; 57(8):1431-8.
5. Strangfeld A, Listing J, Herzer P, et al. Risk of herpes zoster in patients with rheumatoid arthritis treated with anti-TNF-alpha agents. JAMA 2009; 301(7):737-44.
6. McDonald J, Zeringue A, Caplan L, et al. Herpes zoster risk factors in a national cohort of veterans with rheumatoid arthritis. Clin Infect Dis 2009; 48(10):1364-71.
7. Winthrop K, Baddley J, Chen L, et al. Association between the initiation of anti-tumor necrosis factor therapy and the risk of herpes zoster. JAMA 2013; 309(9):887-95.
8. Galloway J, Mercer L, Moseley A, et al. Risk of skin and soft tissue infections (including shingles) in patients exposed to anti-tumor necrosis factor therapy: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis 2013; 72(2):229-34.
9. Rimland D, Moanna A. Increasing incidence in herpes zoster among veterans. Clin Infect Dis 2010; 50(7):1000-5.
10. Oxman M, Levin M, Johnson G, et al. Shingles prevention study group. A vaccine to prevent herpes zoster and post-herpetic neuralgia in older adults. N Engl J Med 2005; 352(22):2271-84.
11. Schmader K, Levin M, Gnann J, et al. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50-59 years. Clin Infect Dis 2012; 54(7):922-8.
12. Schmader K, Oxman M, Levin M, et al. Persistence of the efficacy of zoster vaccine in the shingles prevention study and the short-term persistence substudy. Clin Infect Dis 2012; 55(10):1320-8.
13. Zhang J, Xie F, Delzell E, et al. Association between vaccination for herpes zoster and risk of herpes zoster infection among older patients with selected immune-mediated diseases. JAMA 2012; 308(1):43-9.
14. Tseng H, Chi M, Smith N, et al. Herpes zoster vaccine and the incidence of recurrent herpes zoster in an immunocompetent elderly population. J Infect Dis 2012; 206(2):190-6.
15. Bombardier C, Hazlewood G, Akhavan P, et al. Canadian Rheumatology Association recommendations for the pharmacological management of rheumatoid arthritis with traditional and biologic disease-modifying antirheumatic drugs: Part II safety. J Rheumatol 2012; 39(8):1583-602.
16. Charkoudian LD, Kaiser GM, Steinmetz RL, Srivastava SK. Acute retinal necrosis after herpes zoster vaccination. Arch Ophthalmol 2011; 129(11): 1495-7.
Gordon Dow, MD, FRCPC
Clinical Assistant Professor of Medicine,
Dalhousie University
Section of Infectious Diseases,
The Moncton Hospital
Moncton, New Brunswick |



