Spring 2014 (Volume 24, Number 1)
RA Guidelines: Practice
Patterns of Rheumatologists in Canada Compared to CRA Recommendations for RA
(Part IV)
By Sankalp V. Bhavsar, MD, FRCPC;
on behalf of Carter Thorne, MD, FRCPC, FACP;
Claire Bombardier, MD, FRCPC;
Vivian P. Bykerk, MD, FRCPC;
Glen S. Hazlewood, MD, FRCPC;
Pooneh Akhavan, MD, FRCPC;
Orit Schieir, MSc; and
Sanjay Dixit, MD, FRCPC
Download PDF
In this instalment, we present the results of survey questions pertaining to treatment with biologic disease-modifying antirheumatic drugs (DMARDs) and perioperative care.
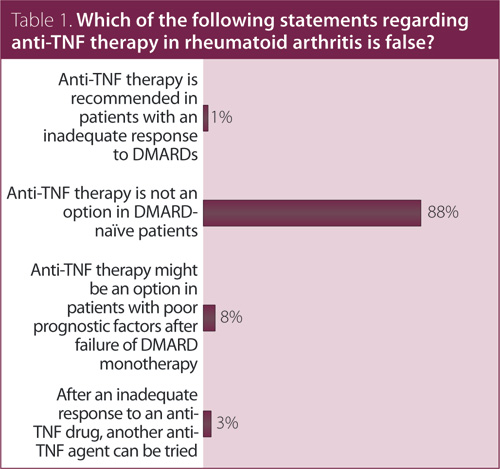
1. Which of the following statements regarding anti-tumor necrosis factor (TNF) therapy in rheumatoid arthritis (RA) is false?
Answer: Anti-TNF therapy is not an option in DMARD-naïve patients. Recommendation/supporting evidence: European League Against Rheumatism (EULAR) 2010,1 Canadian Agency for Drugs and Technologies in Health (CADTH) 2010,2 EULAR 2013.3
Systematic reviews performed by EULAR1 and CADTH2 considered all anti-TNF agents (adalimumab [ADA], certolizumab [CTZ], etanercept [ETN], infliximab [IFX], and golimumab [GOL]) and trials in both DMARD-inadequate responders and methotrexate (MTX)-naïve patients. There is direct randomized controlled trial (RCT) evidence of efficacy for all anti-TNF therapies in patients who have had an inadequate response to MTX. For IFX, ETN, ADA, and GOL, there is also RCT evidence for efficacy in patients who are MTX-naïve. Some patients in these trials were also DMARD-naïve and all patients had early RA with high baseline disease activity. There were no head-to-head trials comparing anti-TNF agents. Although the 2013 update of the EULAR recommendations3 has abandoned the former recommendation that DMARD-naïve patients with poor prognostic markers might be considered for combination therapy of MTX plus a biologic, the exceptional use of a biologic agent in such patients is not precluded.
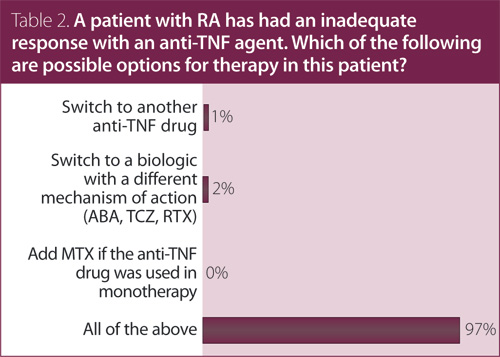
2. A patient with RA has had an inadequate response with an anti-TNF agent. Which of the following are possible options for therapy in this patient?
Answer: All of the above.
Recommendation/supporting evidence: EULAR 2010,1 CADTH 2010,2 EULAR 2013.3
A systematic review of RCTs used to inform the EULAR 2010 guidelines1 provided evidence supporting the efficacy of rituximab (RTX), abatacept (ABA), tocilizumab (TCZ), and GOL in patients who have failed one anti-TNF agent. CADTH2 referred to a health technology assessment performed by the National Institute of Clinical Excellence (NICE) on options for treatment with biologic agents after failure of an anti-TNF; the conclusion was that switching to another anti-TNF may have some benefit based on observational studies. There is also RCT evidence for dose escalation of biologics. We found contradictory evidence for IFX (two trials showing benefit and one showing no benefit) and found evidence against dose escalation of ETN. There was no evidence provided to support dose/interval adjustment of ADA. Current evidence does not suggest a specific agent to be preferable to another TNF inhibitor when there is active disease, despite initial treatment with a TNF inhibitor.
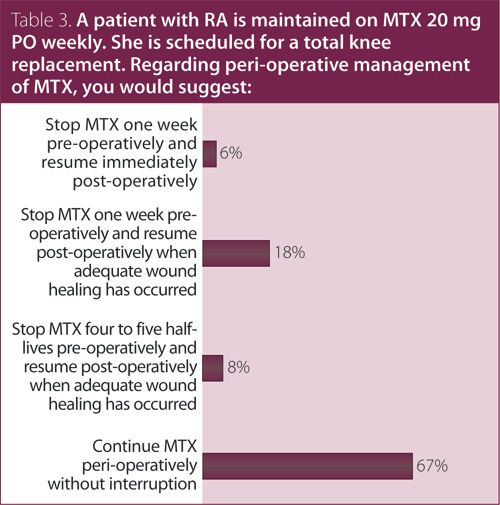
3. A patient with RA is maintained on MTX 20 mg PO weekly. She is scheduled for a total knee replacement. Regarding perioperative management of MTX, you would suggest?
Answer: Continue MTX perioperatively without interruption.
Recommendation/supporting evidence: Visser 2009,4 British Society for Rheumatology (BSR) 2009,5 BSR 2008.6
The BSR5,6 and Visser et al4 referred to evidence from RCT and observational studies that examined outcomes in RA patients who stopped treatment versus those who continued MTX prior to elective orthopedic surgery. The largest RCT of RA patients undergoing elective orthopedic surgery showed a lower rate of postoperative complications, including infection in patients who continued MTX perioperatively (2/88 [2%]) compared to those who discontinued MTX (11/72 [15%]), and fewer RA flares six weeks after surgery (0/88 [0%] vs. 6/72 [8%]). Consistent results were also reported in a smaller RCT of 64 RA patients and in a retrospective cohort study of 122 RA patients. Only two small cohort studies (n = 32 and n = 38, respectively) have reported an increased risk of local infections in RA patients who continued compared to those who discontinued MTX prior to orthopedic surgery.
For further information on these recommendations and the supporting evidence of these results, please consult the CRA RA Guidelines document, available at www.rheum.ca/en/publications/cra_ra_guidelines.
References
1. Smolen JS, Landewe R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis 2010; 69(6):964-75.
2. Canadian Agency for Drugs and Technology in Health. Clinical and economical overview: Biological response modifier agents for adults with rheumatoid arthritis; 2010. Available at www.cadth.ca/media/pdf/TR_RA_Clinical_and_ Economic_Overview_e.pdf.
3. Smolen JS, Landewe R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 2013; 0:1-18.
4. Visser K, Katchamart W, Loza E, et al. Multinational evidence-based recommendations for the use of methotrexate in rheumatic disorders with a focus on rheumatoid arthritis: integrating systematic literature research and expert opinion of a broad international panel of rheumatologists in the 3E Initiative. Ann Rheum Dis 2009; 68(7):1086-93.
5. Chakravarty K, McDonald H, Pullar T, et al. BSR/BHPR guideline for disease-modifying anti-rheumatic drug (DMARD) therapy in consultation with the British Association of Dermatologists. Rheumatology (Oxford) 2008; 47(6):924-5.
6. Luqmani R, Hennell S, Estrach C, et al. British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of rheumatoid arthritis (after the first 2 years). Rheumatology (Oxford) 2009; 48(4):436-9.
Sankalp V. Bhavsar, MD, FRCPC
Rheumatology Fellow,
McMaster University
Hamilton, Ontario
on behalf of Carter Thorne, MD, FRCPC, FACP;
Claire Bombardier, MD, FRCPC;
Vivian P. Bykerk, MD, FRCPC;
Glen S. Hazlewood, MD, FRCPC;
Pooneh Akhavan, MD, FRCPC;
Orit Schieir, MSc; and
Sanjay Dixit, MD, FRCPC |



