Spring 2014 (Volume 24, Number 1)
Tuberculosis Prophylaxis and Biologics Treatment for Rheumatoid Arthritis
By Nicholas M. Baniak, BSc;
Vernon M. Hoeppner, MD, FRCPC; and
Wojciech P. Olszynski, MD, PhD, FRCPC, CCD
Download PDF
Tuberculosis (TB) infection is a prevalent, mostly latent, disease. Being treated with anti-tumor necrosis factor (TNF) inhibitors, a form of biologic therapy used for the treatment of rheumatoid arthritis (RA), is believed to increase the risk of reactivation. Accordingly, RA patients are recommended to go through screening for latent TB prior to initiating biologic therapy. If RA patients are found to have latent TB, it is recommended they initiate TB prophylaxis prior to beginning TNF inhibitor therapy. In the present study, a group of patients positive for latent TB were not provided prophylaxis before commencing TNF inhibitor therapy and were subsequently closely monitored for the development of overt TB symptoms. Of the
213 patients examined, 52% were male and 48% female, with 71% being over the age of 50. Furthermore, 95% of patients had been receiving treatment for longer than one year, with the longest being treated for 10 years. None of the patients showed evidence of active TB while on biologic therapy.
Introduction
A third of the world’s population is infected with TB,1,2 including 4% of the United States population.3 In Canada, certain ethnicities possess higher levels of latent TB infection, such as foreign-born and First-Nations populations. There is an increased risk of TB associated with the use of TNF inhibitors, therapies commonly used for the management of autoimmune disorders, such as RA.3 In a study of over 112,000 Canadian patients with RA, the rate of TB in patients not treated with TNF inhibitors was 2.2/1,000 patients, compared to 2.6/1,000 patients in those treated with TNF inhibitor therapy.4,5
Mycobacteria are facultative intracellular pathogens.1,6 When inhaled, TB bacilli are taken in by alveolar macrophages and encapsulating granulomas are subsequently developed in an attempt to limit the spread of the infectious bacteria.1,6 Since the patient is not able to completely eliminate the pathogens, the resulting granulomas are the characteristic feature of latent pulmonary TB (LTBI).7 Most immune-competent hosts have a sufficiently strong immune response to TB bacteria, limiting these pathogens to the lungs and associated lymph nodes.7,8 The histiocytic transformation and formation of granulomas represents residual infection.6 Disease reactivation occurs when latent bacteria from
pre-existing granulomas are reactivated into an active, virulent state; reactivation is most common when the host immune response weakens or is suppressed.7 Suppression of immune response is a well-known side effect of TNF inhibitor therapy.
The interaction between activated macrophages and interferon-gamma (IFN-y)-secreting lymphocytes is vital to controlling the infection. TNFα, which is released by activated immune cells, also plays an important role in both granuloma formation and maintenance through its effects on expression of adhesion molecules and chemokines.9-14 Therefore, TNF inhibitor treatment may cause the granuloma to fail, allowing TB release and reactivation.11 In a TNF-deficient mouse model, rapid TB infection and subsequent death is observed.7,15
TB screening is recommended for the identification of latent TB infection in patients considering initiating TNF inhibitor therapy for RA,16 as surveys have shown that the incidence of TB is increased following the initiation of TNF inhibitor therapy.11 Therefore, it is imperative that LTBI is identified and treated prior to initiating TNF inhibitor therapy to minimize the risk of reactivation.17
The 2012 American College of Rheumatology (ACR) treatment recommendations for those with latent TB (positive TST [tuberculin skin test] and negative chest X-ray [CXR]) are to take prophylactic medication before initiating any biologic medicine, such as a TNF inhibitor.14 The treatment for LTBI is isoniazid (INH) 5 mg/kg (up to 300 mg) once daily or 15 mg/kg (up to 900 mg) twice weekly18 for nine months.1,19-21 However, as with any medication, the risk of side effects when taking the TB prophylaxis, especially hepatotoxicity, must be weighed against the benefit of preventing reactivation of TB.22-24
In Saskatoon, Canada, RA patients with a positive TB skin test, but no other overt signs of TB, are initiated on TNF inhibitor therapy (after failing disease modifying anti-rheumatic drugs [DMARDs]) without prophylaxis and are monitored closely at the Saskatoon TB clinic. Patients with positive TSTs were compared to patients on TNF inhibitors with a negative TST to see if reactivation rates of TB differed between the two groups. The literature has shown no consistent pattern of serious TB infection risk associated with the use of TNF inhibitors.25 In this study, it was hypothesized that the patients who were TST positive were not at any increased risk of TB reactivation by taking TNF inhibitors.
The objective of this study was to determine if patients who test positive for TB have a significantly different risk of reactivation when not provided prophylaxis as compared to those who are prophylactically treated.
Materials and Methods
The cohort for this investigation consisted of all patients receiving biologic therapy at the office of a private urban rheumatology clinic and from the Royal University Hospital (RUH) in Saskatoon, Canada. Patient medical charts (n = 213) were reviewed from 2002-2012 for the following variables: age, gender, type of biologic therapy, types of DMARDs (particularly prednisone), and signs of active TB.
Patients were divided into two groups: those with either a positive TST
(> 5 mm) or those with a negative TST (≤ 5 mm). Of the patients with a positive TST, 26 had RA, eight had ankylosing spondylitis (AS), and five had psoriatic arthritis (PsA). Of the patients with a negative TST, 127 had RA, 29 had AS, 16 had PsA, and two had inflammatory bowel disease (IBD) arthropathy. Total time that patients were receiving biologics was measured in months and patient-years due to the unequal time in follow up in the different patients. There was no minimum amount of time that a patient had to be receiving biologics to be included, with the minimum time being three weeks for one patient. However, only two patients were receiving biologics for less than one year. No patient received TB prophylaxis.
Results
No evidence of TB reactivation occurred in either patient group. All of the patients included in this survey had moderate to severe RA as evidenced by having symptoms despite multiple types of DMARD treatments, and had thus been administered TNF inhibitor therapy. There were
39 patients with a positive TST currently receiving a TNF inhibitor and 174 patients with a negative TST receiving a TNF inhibitor. The majority of patients in both groups were over the age of 50 and were concurrently administered DMARDs (Table 1). Despite TNF inhibitor therapy, none of the patients showed evidence of active TB in follow up.
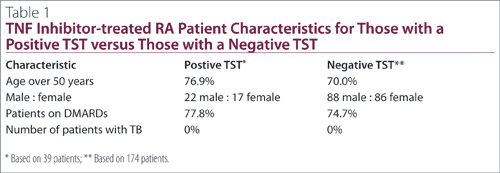
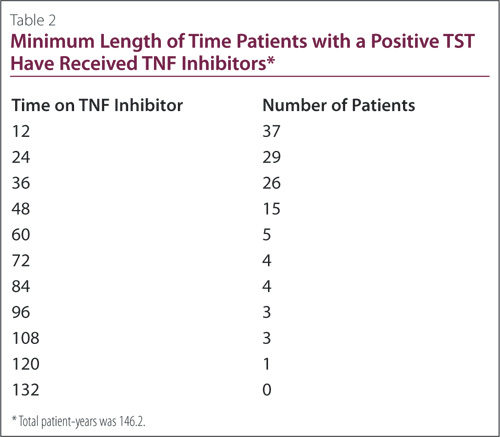
All but two of the positive TST patients received TNF inhibitors for at least 12 months, with the two receiving therapy for 6.9 and 0.7 months
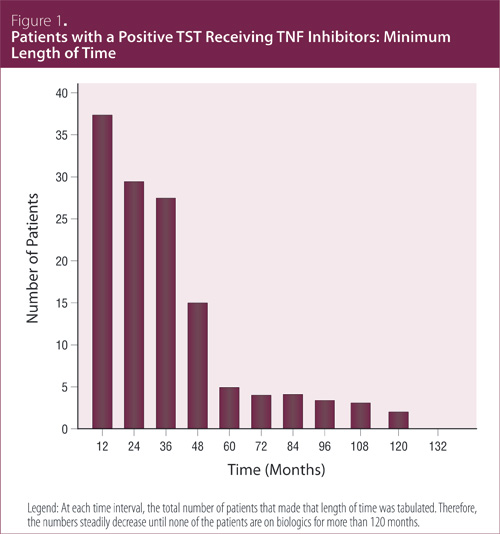
(Table 2). At each time interval, the total number of patients that made that length of time was tabulated. The numbers steadily decreased; none of the patients were treated with biologics for more than 120 months
(Figure 1). In total, the positive TST patients accumulated 146 patient-years of TNF inhibitor therapy and the negative TST patients 746 patient-years.
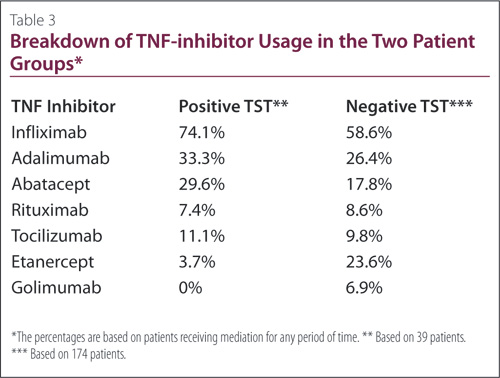
Patients were treated with a range of TNF inhibitors; it was common for the patients to have been administered more than one form of TNF inhibitor therapy over the course of their treatment (Table 3). A total of 74% of positive TST patients and 59% of negative TST patients were receiving infliximab at some point in time, which was the most commonly-used TNF inhibitor. Of the remaining agents, the most common to least commonly administered were adalimumab, abatacept, rituximab, tocilizumab, etanercept, and golimumab. None of the TST-positive patients had taken golimumab.
Of the 39 patients with a positive TST, 17 (44%) had a history of prednisone use, while 59 of the 174 patients (34%) with a negative TST had a history of prednisone use.
Discussion
Despite using TNF inhibitors without TB prophylaxis in a population considered at risk for TB reactivation, that being patients with a positive TST, no cases of reactivation were observed. Investigations have reported that the majority of LTBI reactivations due to TNF inhibitor administration occur in the early phase of treatment,4,11,26-28 with the median time of reactivation between 12-17 weeks.4,11 In this chart review, all but two of the patients received TNF inhibitors for more than
12 months. Furthermore, all of the patients were monitored closely at the Saskatoon TB clinic for two years after initiating TNF inhibitor therapy. If TB were to occur, the reactivations would have been most likely within that two-year window.11,26
The level of increased risk of TB reactivation among the different biologics has been reported to differ. In one study, incident rates of TB reactivation were found to be highest with infliximab (1.5/1,000 patient-years), followed by adalimumab (0.9/1,000 patient-years), and then etanercept (0.5/1,000 patient-years).17 Other reports also placed infliximab as the most at risk for causing TB reactivation, followed by adalumimab, then etanercept.26 Some studies have found a three- to four-fold higher risk when on infliximab or adalimumab as compared to etanercept;27-29 while other investigations have claimed there to be no difference between infliximab, adalumimab, and etanercept with respect to TB reactivation.25
There has been no consistent pattern of serious TB infection risk associated with the use of TNF inhibitors.25 Adalimumab was launched after the risk of TB had emerged and screening initiated, which may account for some of the over-reported rates of TB in patients on adalimumab, as a consequence of increased vigilance.25 The mechanism of action of rituximab is not a concern for TB reactivation as while receiving TNF inhibitors.30 In fact, there are no reported cases to date with patients being treated with rituximab,30 nor have increased rate of TB been shown with tocilizumab.28 However, since none of the patients developed active TB in our study, there does not appear to be an increased risk with any of the medications used.
As mentioned, the preferred regimen for treating LTBI is nine months of INH daily.1,19,21,22 The efficacy of IHN has been reported as 60% for six months of daily INH (> 80% completion),31,32 and 90% for nine months of daily INH.33 The completion rate is, however, very low, with one study showing persistence as low as 39%,34 and other reports claiming rates between 50% and 60%.35,36
There are considerable risks associated with TB prophylaxis. The most serious side effect of INH is toxic hepatitis.23 Hepatic adverse effects from INH range from a mild increases in aminotransferases (10%-20%) to overt hepatitis, which is rare.24 Risk factors include age over 35 years, being female, baseline elevation of aspartate aminotransferase (AST), and the concurrent use of alcohol.26,29,37 With over one million patients treated with INH since 1991, the incidence of INH-associated liver injury has been estimated at 1/1,000 patients,19 hospitalization rates have been reported at 0.1 to 0.2/1,000, and mortality rates of 0.0 to 0.3/1,000.6,20,37 In public health clinic studies, the incidence of INH hepatotoxicity has varied between 0.1%24 and 4%.38 The differences is perhaps due to age of the population or definition of hepatotoxicity in these studies.39 Another study found a rate of 5.63 hepatotoxic events per 1,000 patients, with higher rates associated with patients over the age of 50.39 It should be noted that in this database study, only 41% of patients were to found to have completed three months of INH therapy, and only 22% six months of therapy.39 The toxicity may have been higher in some instances if the compliance had been higher.39 In one trial, 53% of the 255 patients that completed the nine months of INH therapy reported some symptoms during the treatment.2 In the same trial, hepatotoxicity accounted for 40% of those patients permanently discontinued from the treatment.2
There are competing risks when considering treatment: TNF inhibitors and the reactivation of TB versus INH toxicity and compliance. If the annual risk of TB is greater than the risk of drug induced hepatitis, then prophylaxis should be received.22 Conversely, if the risk of hepatitis is greater, then patients should not receive prophylaxis, but rather be monitored closely, having any symptoms that develop investigated quickly and diagnosed early.22 When risk outweighs benefit, patients with an abnormal chest X-ray consistent with past TB (or prior extra pulmonary TB that has been adequately treated in the past) can begin TNF inhibitor treatment while being monitored clinically every three months.22 If no adequate treatment was received, then the risk-benefit analysis favors chemotherapy.22
To illustrate the point, consider treatment for an average RA patient in Saskatchewan. The incidence of TB in Canada is 5.1/100,000, while in Saskatchewan it is somewhat higher at 6.2 /100,000.31 Specific incidences of TB in Saskatchewan are less than 1/100,000 for Caucasians, 43/100,000 for status First Nations, 23/100,000 for Metis, and 17/100,000 for foreign-born Canadians.31 The progression from latent infection to active TB has a universal estimate of a 10% incidence with a positive TST.31 In Saskatchewan, the rates are 0.8% and 6.9% for White-European and Status First Nation individuals, respectively.31 In order to make a risk calculation, the annual risk of TB should be multiplied by five (due to the increased risk caused by TNF inhibitor therapy) to account for TNF inhibitor medications,22 which would be divided by the risk of INH hepatitis.31 A ratio of less than one indicates observation is best, while a ratio of more than one would indicate that prophylaxis is preferred.31 The incidence would be determined by reviewing local epidemiology for the patient group and the toxicity as 278/100,000 people.31 For example, a Caucasian in Saskatchewan would have a risk of ([1/100,000]*5)/ ([278/100,000]), which would be 0.02, strongly favoring observation.31 Even for a Status First Nation patient, the ratio would be 0.8 ([43 cases/100,000 people]*5)/
([278 cases/100,000 people]).30
In this study, 76.2% of the RA patients treated with TNF inhibitors with a positive TST were over the age of 50 years, they would have been at a higher risk of toxicity. The risk-benefit ratio was less than one for all of the patients as well. Therefore, all of the patients theoretically would be safer not receiving prophylaxis.
In addition to being safer for the patient, not having to give prophylaxis would be beneficial for the health care system in terms of cost. In a study using financial information from Montreal, Canada, the estimated cost of treating one patient with nine months of INH was $1,073 if no symptoms developed, and $1,131 if symptoms developed but therapy was still completed.2 Costs were attributable to routine visits, therapeutic agents, pharmacy charges, routine testing, and unscheduled visits. The costs of evaluation and management of specific adverse events varied from $668 to $1,249, depending on the severity of the adverse event.2 Although medication is inexpensive for LTBI, the total costs are high because close monitoring is imperative due to the risk of drug-induced hepatitis.2
The exact risk of morbidity of TB associated with corticosteroid therapy is unknown, but therapy with them is a well-known risk factor for TB.40 Reactivation of TB after patients were administered corticosteroids has been documented.41-43 Corticosteroids have an immunosuppressive effect, which can promote TB reactivation, therefore, careful observation of patients taking steroids is required.42,44 However, there has also been no relationship found between total dose or duration and risk.42
One of the limitations of this study was the small number of patients (39) with a positive TST, making it impossible to compare results with previous studies. With an expected risk of TB in TST-positive patients on TNF inhibitors of 2.6/1,000, the study would need about 400 patients to show one case of TB. There are not enough people in Saskatchewan to provide sufficient numbers. As far as we know, Saskatchewan is the only place providing biologics to TST-positive patients without receiving TB prophylaxis prior to therapy. Consequently, all the patients must come from Saskatchewan, making it very difficult to attain sufficient numbers of patients.
Although the numbers of patients in this study are not sufficient to compare the levels of risks with other studies, it shows that none of the RA patients on TNF inhibitors had reactivation of their TB.
Conclusion & Summary
In this investigation we demonstrated that RA patients on TNF inhibitors who did not receive prophylaxis are not at risk for TB reactivation. Although we could not fully answer the objective question of whether patients are at increased risk by not being on prophylaxis, the study acts as a probing study into the possibility of treating RA with biologics in patients with latent TB without the need for TB prophylaxis. Between
400 and 5,000 patients would be needed to objectively determine that there is no increased risk, but it is at least suggested. More investigations are needed, along with more follow up to provide more data.
We would like to acknowledge K. Shawn Davison for his contributions to editing.
References
1. Winthrop KL. Update on tuberculosis and other opportunistic infections associated with drugs blocking tumour necrosis factor {alpha}. Ann Rheum Dis 2005; 64 Suppl 4:iv29-30.
2. Aspler A, Long R, Trajman A, et al. Impact of treatment completion, intolerance and adverse events on health system costs in a randomised trial of 4 months rifampin or 9 months isoniazid for latent TB. Thorax 2010; 65(7):582-7.
3. Bennett DE, Courval JM, Onorato I, et al. Prevalence of tuberculosis infection in the United States population: the national health and nutrition examination survey, 1999–2000. Am J Respir Crit Care 2008; 177(3):348-55.
4. Patkar NM, Teng GG, Curtis JR, et al. Association of infections and tuberculosis with antitumor necrosis factor alpha therapy. Curr Opin Rheumatol 2008; 20(3):320-6.
5. Brassard P, Kezouh A, Suissa S. Antirheumatic drugs and the risk of tuberculosis. Clin Infect Dis 2006; 43(6):717-22.
6. Villiger PM, Zellweger JP, Möller B. Novel screening tools for latent tuberculosis: time to leave an old friend? Curr Opin Rheumatol 2009; 21(3):238-43.
7. Gupta A, Kaul A, Tsolaki AG, et al. Mycobacterium tuberculosis: immune evasion, latency and reactivation. Immunobiology 2012; 217(3):363-74.
8. Gomez JE, McKinney JD, M. tuberculosis persistence, latency, and drug tolerance. Tuberculosis (Edinb) 2004; 84(1-2):29-44.
9. Tufariello JM, Chan J, Flynn JL. Latent tuberculosis: mechanisms of host and bacillus that contribute to persistent infection. Lancet Infect Dis 2003; 3(9):578-90.
10. Gardam MA, Keystone EC, Menzies R, et al. Anti-tumour necrosis factor agents and tuberculosis risk: mechanisms of action and clinical management. Lancet Infect Dis 2003; 3(3):148-55.
11. Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med 2001; 345(15):1098-104.
12. Settas LD, Tsimirikas G, Vosvotekas G, et al. Reactivation of pulmonary tuberculosis in a patient with rheumatoid arthritis during treatment with IL-1 receptor antagonists (anakinra). J Clin Rheumatol 2007; 13(4):219-20.
13. Lin PL, Plessner HL, Voitenok NN,et al. Tumor necrosis factor and tuberculosis. J Investig Dermatol Symp Proc 2007; 12(1):22-5.
14. Lin PL, Flynn JL. Understanding latent tuberculosis: a moving target. J Immunol 2010; 185(1):15-22.
15. Botha T, Ryffel B. Reactivation of latent tuberculosis infection in TNF- deficient mice. J Immunol 2003; 171(6):3110-8.
16. Singh JA, Furst DE, Bharat A, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012; 64(5):625-39.
17. Dixon WG, Watson K, Lunt M, et al, on behalf of the British Society for Rheumatology Biologics Register. Rates of serious infection, including site-specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving anti–tumor necrosis factor therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum 2006; 54(8):2368-76.
18. American Thoracic Society (ATS) and Centers for Disease Control Prevention (CDC). Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med 2000; 161(4 Pt 2):S221-47.
19. CDC. Targeted tuberculin testing and treatment of latent tuberculosis infection. MMWR Mortal Morb Wkly Rep 2000; 49(6):1-51.
20. Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a joint statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med 2000; 161(4 pt 2):S221-47.
21. Long R. Canadian tuberculosis standards. 6th ed. Ottawa, ON, Canada: Canadian Lung Association & Public Health Agency of Canada, 2007.
22. British Thoracic Society Standards of Care Committee. BTS recommendations for assessing risk and for managing Mycobacterium tuberculosis infection and disease in patients due to start anti-TNF-alpha treatment. Thorax 2005; 60(10):800-5.
23. da Rocha Castelar Pinheiro G. Rheumatoid arthritis and tuberculosis in the tumor necrosis factor inhibitors era: observations from Brazil. J Clin Rheumatol 2005; 11(6):344-6.
24. Nolan CM, Goldberg SV, Buskin SE. Hepatotoxicity associated with isoniazid preventive therapy: a 7-year survey from a Public Health Tuberculosis Clinic. JAMA 1999; 281(11):1014-8
25. Dixon WG, Watson K, Lunt M, et al. British Society for Rheumatology Biologics Register. Rates of serious infection, including site-specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving anti-tumor necrosis factor therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum 2006; 54(8):2368-76.
26. Szeto T, Peterson J, Silva F. A case of tuberculous peritonitis in the United States in a patient with rheumatoid arthritis treated with adalimumab. J Clin Rheumatol 2010; 16(3):135-7.
27. Tubach F, Salmon D, Ravaud P, et al. Risk of tuberculosis is higher with anti-tumor necrosis factor monoclonal antibody therapy than soluble tumor necrosis factor receptor therapy. Arthritis Rheum 2009; 60(7):1884-94.
28. Campbell L, Chen C, Bhagat SS, et al. Risk of adverse events including serious infections in rheumatoid arthritis patients treated with tocilizumab: a systematic literature review and meta-analysis of randomized controlled trials. Rheumatology (Oxford) 2011; 50(3):552-62.
29. Jung N, Owczarczyk K, Hellmann M, et al. Efficacy and safety of rituximab in a patient with active rheumatoid arthritis and chronic disseminated pulmonary aspergillosis and history of tuberculosis. Rheumatology (Oxford) 2008; 47(6):932-3.
30. Burr ML, Malaviya AP, Gaston JH, et al. Rituximab in rheumatoid arthritis following anti-TNF-associated tuberculosis. Rheumatology (Oxford) 2008; 47(5):738-9.
31. Hoeppner V, Olszynski W. Prophylactic treatment of T infection: risks vs. benefits. Personal communications, 2006.
32. Smieja MJ, Marchetti CA, Cook DJ, et al. Isoniazid for preventing tuberculosis in non-HIV infected persons (Cochrane Review). Cochrane Database Syst Rev 2000; (2):CD001363.
33. Comstock GW. How much isoniazid is needed for prevention of tuberculosis among immunocompetent adults? Int J Tuberc Lung Dis 1999; 3(10):847-50.
34. Horsburgh CR Jr, Goldberg S, Bethel J, et al. Tuberculosis Epidemiologic Studies Consortium. Latent TB infection treatment acceptance and completion in the United States and Canada. Chest 2010; 137(2):401-9.
35. Page KR, Sifakis F, Montes de OR, et al. Improved adherence and less toxicity with rifampin vs isoniazid for treatment of latent tuberculosis: a retrospective study. Arch Intern Med 2006; 166(17):1863-70.
36. Jasmer RM, Nahid P, Hopewell PC. Latent tuberculosis infection. N Engl J Med 2002; 347(23):1860-6.
37. Bruns H, Meinken C, Schauenberg P, et al. Anti-TNF immunotherapy reduces CD8+ T cell-mediated antimicrobial activity against Mycobacterium tuberculosis in humans. J Clin Invest 2009; 119(5):1167-77.
38. McNeill L, Allen M, Estrada C, Cook P. Pyrazinamide and rifampin vs isoniazid for the treatment of latent tuberculosis: improved completion rates but more hepatotoxicity. Chest 2003; 123(1):102-6.
39. Fountain FF, Tolley E, Chrisman CR, et al. Isoniazid hepatotoxicity associated with treatment of latent tuberculosis infection: a 7-year evaluation from a public health tuberculosis clinic. Chest 2005; 128(1):116-23.
40. Bass JB Jr, Farer LS, Hopewell PC, et al. Treatment of tuberculosis and tuberculosis infection in adults and children. American Thoracic Society and The Centers for Disease Control and Prevention. Am J Respir Crit Care Med 1994; 149(5):1359-74.
41. Haanaes OC, Bergmann A. Tuberculosis emerging in patients treated with corticosteroids. Eur J Respir Dis 1983; 64(4):294-7.
42. Kobashi Y, Matsushima T. Clinical analysis of pulmonary tuberculosis in association with corticosteroid therapy. Intern Med 2002; 41(12):1103-10.
43. Sahn SA, Lakshminarayan S. Tuberculosis after corticosteroid therapy. Br J Dis Chest 1976; 70(3):195-205. 44. Senderovitz T, Viskum K. Corticosteroids and tuberculosis. Respir Med 1994; 88(8):561-5.
Nicholas M. Baniak, BSc
College of Medicine,
University of Saskatchewan
Saskatoon, Saskatchewan
Vernon M. Hoeppner, MD, FRCPC
Professor and Head,
Department of Medicine
College of Medicine,
University of Saskatchewan
Saskatoon, Saskatchewan
Wojciech P. Olszynski, MD, PhD, FRCPC, CCD
Clinical Professor of Medicine Director,
Saskatoon Osteoporosis and Arthritis Infusion Centre
Honorary Consul of the Republic of Poland in Saskatoon
Saskatoon, Saskatchewan |



