Fall 2014 (Volume 24, Number 3)
RA Guidelines: Practice Patterns of Rheumatologists in Canada Compared to the CRA Recommendations for RA
(Part VI)
By Sankalp V. Bhavsar, MD, FRCPC;
on behalf of Carter Thorne, MD, FRCPC, FACP;
Claire Bombardier, MD, FRCPC;
Vivian P. Bykerk, MD, FRCPC;
Glen S. Hazlewood, MD, FRCPC;
Pooneh Akhavan, MD, FRCPC;
Orit Schieir, MSc; and
Sanjay Dixit, MD, FRCPC
Download PDF
In this sixth and final instalment, we present the results of survey questions pertaining to malignancy.
1. A patient with rheumatoid arthritis (RA) maintained on etanercept and methotrexate (MTX) now has newly diagnosed lung cancer and will be starting radiation and chemotherapy. Regarding etanercept, you would suggest:
Answer: Stop etanercept.
Recommendation/supporting evidence: Not applicable. Consensus recommendation.
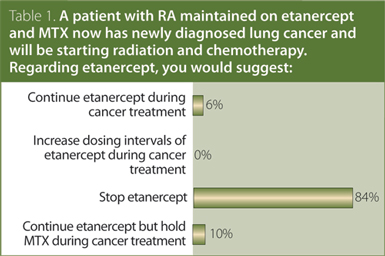
No identified evidence addressed the use of traditional or biologic disease-modifying antirheumatic drugs (DMARDs) in RA patients with active malignancy. The CRA recommendation is that, in general, RA patients with active malignancy should have their treatment with traditional and biologic DMARD delayed or withheld while they are receiving chemotherapy or radiotherapy. Treatment decisions should be made on a case-by-case basis in conjunction with a cancer specialist and the patient.
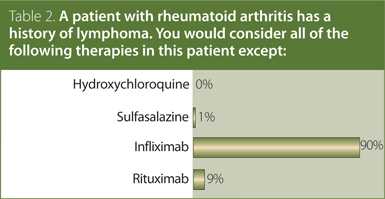
2. A patient with RA has a history of lymphoma. You would consider all of the following therapies in this patient except:
Answer: Infliximab.
Recommendation/supporting evidence: Not applicable. Consensus recommendation.
MTX/DMARD: One nested case-control study examined the risk of incident lymphoma (not recurrence) in RA patients treated with MTX compared to RA patients treated with other DMARDs and reported a small but not statistically significant increased risk associated with MTX (RR 1.23, 95% CI 0.97–1.57). In that study, no increased risk of lymphoma was associated with use of leflunomide,
sulfasalazine, antimalarials, or gold. Four studies examined risks of lymphoproliferative disease in patients with RA treated with MTX and/or other DMARDs relative to the general population; all showed an increased risk (non-Hodgkin’s lymphoma: standardized incidence ratio [SIR] = 5.1, 95% CI 2.2–10, SIR = 5.4, 95% CI 1.1–15.7; any lymphoproliferative disease:
SIR = 3.8, 95% CI 2.2–6.2; lymphoma: SIR = 1.7, 95% CI 0.9–3.2).
Biologics: A Cochrane network meta-analysis of randomized trials examining the safety of biologic therapy (excluding tocilizumab) did not observe an increased risk of lymphoma associated with any biologic therapy (OR 0.53, 95% CI 0.17–1.66), although data were limited to very few events. Similarly, four observational studies compared the risk of lymphoma in RA patients treated with anti-tumor necrosis factor (TNF) therapy relative to RA patients who were biologic-naïve; none showed an increased risk associated with anti-TNF therapy, although small numbers of malignancies and lack of precision around this study estimates precluded definitive conclusions (RR 1.4, 95% CI 0.8–2.1; HR 1.1, 95% CI 0.5–2.4; OR 1.0, 95% CI 0.6–1.8; and HR 5.0, 95% CI 0.9–27.9). Eight studies reported an increased risk of lymphoma in RA patients treated with anti-TNF compared to the general population, among which one study reported a higher risk associated with adalimumab or infliximab relative to etanercept (adalimumab: OR 4.7, 95% CI 1.3–17.7; infliximab: OR 4.1, 95% CI 1.4–12.5). In an open-label extension of a randomized, controlled trial, no increased risk of lymphoma was observed in RA patients treated with abatacept relative to RA patients treated with traditional DMARDs.
The CRA recommendation is that hydroxychloroquine, sulfasalazine, and rituximab may be used in RA patients with a history of lymphoma. Treatment with anti-TNF therapy is not recommended, and treatment with other traditional and biologic DMARD should be used with caution.
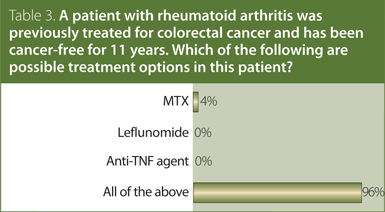
3. A patient with RA was previously treated for colorectal cancer and has been cancer-free for 11 years. Which of the following are possible treatment options in this patient?
Answer: All of the above.
Recommendation/supporting evidence: Not applicable. Consensus recommendation.
MTX/DMARD: Treatment with specific traditional DMARDs was not associated with statistically significant increased risk of lung cancer in a nested case-control study within an administrative claims database. Two studies reported an increased risk of lung cancer in RA patients treated with MTX relative to the general population (SIR 2.9, 95% CI 1.6–4.8; and SIR 3.5, 95% CI 1.4–7.1), one of which also reported an increased risk of melanoma (SIR 3.0, 95% CI 1.2–6.2).
Biologics: RA patients treated with anti-TNF agents were not at increased risk for solid tumors relative to RA patients treated with traditional DMARD in four studies, although one study found an increased risk of melanoma associated with infliximab (OR 2.6, 95% CI 1.0–6.7) and etanercept (OR 2.4, 95% CI 1.0–5.8). Treatment with anti-TNF therapy was not associated with an increased risk for solid tumors relative to the general population in three studies; however, in an analysis of specific types of solid malignancies, one study reported an increased risk of lung cancer and a separate study reported a non-significant trend toward an increased risk for smoking-related cancers (SIR 2.2, 95% CI 0.7–5.1). An increased risk of smoking-related cancers and a decreased risk of breast cancer have also been reported in RA patients not receiving anti-TNF therapy relative to the general population. Abatacept was not associated with an increased risk of solid tumors relative to RA patients receiving traditional DMARDs or the general population in an open-label extension study.
The CRA recommendation is that, in RA patients with a history of solid malignancy, traditional DMARDs may be used. Treatment with biologic DMARDs should be used with caution.
For further information on these recommendations and the supporting evidence of these results, please consult the CRA RA Guidelines document, available at www.rheum.ca/en/publications/cra_ra_guidelines. The previous installations of these Guidelines can be viewed at craj.ca.
Sankalp V. Bhavsar, MD, FRCPC
Vasculitis Fellow, McMaster University,
Hamilton, Ontario
on behalf of Carter Thorne, MD, FRCPC, FACP;
Claire Bombardier MD, FRCPC;
Vivian P. Bykerk, MD, FRCPC;
Glen S. Hazlewood, MD, FRCPC;
Pooneh Akhavan, MD, FRCPC;
Orit Schieir, MSc; and
Sanjay Dixit, MD, FRCPC
|



