Winter 2013 (Volume 23, Number 4)
Capillaroscopy in Rheumatology
By Geneviève Gyger, MD, FRCPC; and
Marie Hudson, MD, MPH, FRCPC
Download PDF
A 40-year-old woman is referred to the rheumatology clinic for nailfold capillaroscopy. She has had Raynaud’s phenomenon for
10 years, has no other relevant medical history, and is a non-smoker. She has three healthy children and no history of miscarriages. She does not take any medication. Family history is unremarkable, including the absence of Raynaud’s phenomenon in any relatives. On history, she denies heartburn, shortness of breath, arthritis, or any other symptoms of connective tissue diseases. The physical exam is negative for sclerodactyly, neck sign, increased peribuccal folds, or skin thickening; however, she has two telangiectasias on her lower inner lip. Her anti-nuclear antibody was positive (1:160), with an anti-centromere pattern. Antibodies to extractable nuclear antigen, including anti-topoisomerase I antibodies, were negative. Nailfold videocapillarocopy was performed (DS Medica, 200x magnification) and showed an active scleroderma pattern (Figure 1).
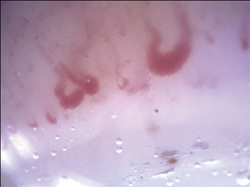
Figure 1. Giant capillaries are the hallmark of the scleroderma pattern. The presence of even one giant capillary is never normal and is characteristic of the scleroderma pattern. Other features of the scleroderma pattern include ectasias, capillary hemorrhages, capillary loss, neoangiogenesis, and disorganisation. More than 95% of SSc patients will have this pattern on videocapillaroscopy. Represented are two giant capillaries, one hemorrhage, and mildly diminished capillary density.
Does This Patient Have Scleroderma?
According to epidemiologic studies, the prevalence of Raynaud’s phenomenon ranges from 2% to 22%, of which roughly 15% is associated with systemic sclerosis (SSc).1-2 Which patients with Raynaud’s phenomenon are likely to progress to SSc? A twenty-year prospective study of 586 patients with Raynaud’s phenomenon showed that abnormal nailfold capillaroscopy at baseline, in the presence of an SSc-specific antoantibody (anti-centromere protein B [CENP-B], anti-topoisomerase I, anti-Th/To, or anti-RNA polymerase III), were excellent predictors for the development of definite SSc, whereas their absence practically ruled out this outcome. Indeed, subjects with both abnormalities at baseline were 60 times more likely to develop SSc compared to patients without these predictors; 80% of the patients with both abnormalities developed SSc over 20 years of follow-up. In contrast, only 2% of patients with Raynaud’s phenomenon with normal capillaroscopy and absent SSc-specific auto-antibodies at baseline developed definite SSc in follow up.2
This landmark study2 provided validation for the criteria proposed for early SSc in 2001 by Leroy and Medsger,3 that included Raynaud’s phenomenon, SSc-specific autoantibodies (anti-centromere, anti-topoisomerase I, anti- fibrillarin, anti-PM/Scl or anti-RNA polymerase I or III) and a scleroderma pattern on capillaroscopy. Those criteria increased the sensitivity of the 1980 American College of Rheumatology (ACR) preliminary criteria4 for limited SSc from 33% to 92%.5 Nevertheless, we should keep in mind that 20% of the patients who fulfill those criteria will not develop SSc, at least with 20 years of follow-up.
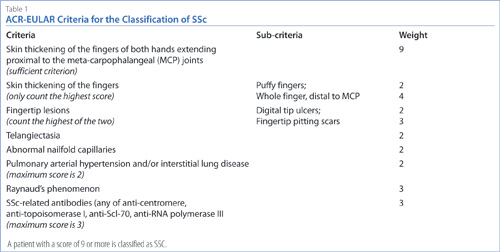
In 2012, an ACR-European League Against Rheumatism (EULAR) committee was established to develop new classification criteria for SSc (Table 1).6 These criteria were expanded to include abnormal nailfold capillaries. A subject with a score of 9 or more is classified as having SSc. The case-study patient described above fulfills the criteria for definite SSc according to the new ACR-EULAR criteria. She has Raynaud’s phenomenon (2 points), anti- centromere antibodies (3 points), SSc-capillaroscopy pattern (2 points), and telangiectasias (2 points), for a total of 9 points. Capillaroscopy was therefore a very useful tool to make a diagnosis of SSc in the case of this patient.
Suggested Work-up
The next step in her investigation should be to determine whether internal organs are involved, including esophageal transit or barium swallow to rule out esophageal dysmotility, echocardiography to measure pulmonary arterial pressures, and chest x-ray and pulmonary function tests to rule out interstitial lung disease. Hand x-rays could also be considered to determine the presence of calcinosis or acro-osteolysis. This patient had a normal work-up, baring the fact that her esophageal transit was compatible with moderate esophageal dysmotility.
Treatment
The treatment for Raynaud’s phenomenon remains symptomatic. Non-pharmacologic interventions include smoking cessation, warm clothes, and minimizing cold exposure. Calcium channel blockers are used as first-line treatment when pharmacotherapy is considered.7 Proton-pump inhibitors (PPIs) should be considered, even in asymptomatic patients, to prevent complications resulting from gastric reflux. Such complications may include esophagitis, esophageal strictures and Barrett’s esophagus. The importance of aggressive treatment of esophageal dysmotility has been highlighted by recent studies which showed a relationship between asymptomatic micro-aspiration secondary to acid reflux and interstitial lung disease in SSc.8-9 Of note, 70% to 90% of SSc patients have esophageal involvement, and 50% of these patients are asymptomatic.7
Conclusion
Nailfold capillaroscopy is a rapid, non-invasive, and easy-to-perform test that should be considered as part of the evaluation of individuals with Raynaud’s phenomenon, to facilitate the diagnosis of SSc, and to reassure those with negative autoantibodies and normal capillaries. The devices presently available for nailfold capillaroscopy include the dermatoscope (10X magnification), ophthalmoscope (20X magnification), widefield microscope (50X magnification), and videocapillaroscope (200X magnification). Although the dermatoscope and ophthalmoscope are available in the clinic, the better resolution of widefield microscopy and videocapillaroscopy is preferable to identify all of the features of the SSc-pattern. The diagnostic and prognostic value of capillaroscopy in other rheumatologic diseases is still being investigated, in particular in the myositides where a scleroderma-like pattern has been described. No specific pattern for other connective tissue diseases has been described. The presence of the SSc-pattern in those diseases suggests an overlap with SSc.
References
1. Spencer-Green G. Outcomes in primary Raynaud phenomenon: a meta-analysis of the frequency, rates, and predictors of transition to secondary diseases. Arch Intern Med 1998; 158(6):595-600.
2. Koenig M, Joyal F, Fritzler MJ, et al. Autoantibodies and microvascular damage are independent predictive factors for the progression of Raynaud's phenomenon to systemic sclerosis: a twenty-year prospective study of 586 patients, with validation of proposed criteria for early systemic sclerosis. Arthritis Rheum 2008; 58(12):3902-12.
3. LeRoy EC & Medsger TA Jr. Criteria for the classification of early systemic sclerosis. J Rheumatol 2001; 28(7):1573-6.
4. Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum 1980; 23(5):581-90.
5. Lonzetti LS, Joyal F, Raynauld JP, et al. Updating the American College of Rheumatology preliminary classification criteria for systemic sclerosis: addition of severe nailfold capillaroscopy abnormalities markedly increases the sensitivity for limited scleroderma. Arthritis Rheum 2001; 44(3):735-6.
6. Van den Hoogen F, Khanna D, Fransen J, et al. Classification criteria for systemic sclerosis: An ACR-EULAR Collaborative Initiative. Arthritis Rheum 2013 (conditionally accepted).
7. Herrick AL. Contemporary management of Raynaud's phenomenon and digital ischaemic complications. Curr Opin Rheumatol 2011; 23(6):555-61.
8. Savarino E, Bazzica M, Zentilin P, et al. Gastroesophageal reflux and pulmonary fibrosis in scleroderma: a study using pH-impedance monitoring. Am J Respir Crit Care Med 2009; 179(5):408-13.
9. Christmann RB, Wells AU, Capelozzi VL, et al. Gastroesophageal reflux incites interstitial lung disease in systemic sclerosis: clinical, radiologic, histopathologic, and treatment evidence. Semin Arthritis Rheum 2010; 40(3):241-9.
Geneviève Gyger, MD, FRCPC
Assistant Professor of Medicine,
McGill University,
Division of Rheumatology,
Jewish General Hospital
Montreal, Quebec
Marie Hudson, MD, MPH, FRCPC
Assistant Professor of Medicine,
McGill University
Division of Rheumatology,
Jewish General Hospital
Lady Davis Institute for Medical Research
Montreal, Quebec |



