Winter 2013 (Volume 23, Number 4)
RA Guidelines: Practice Patterns of Rheumatologists in Canada Compared to CRA Recommendations for RA
(Part III)
By Sankalp Bhavsar, MD, FRCPC; on behalf of Carter Thorne, MD, FRCPC, FACP;
Claire Bombardier, MD, FRCPC; Vivian P. Bykerk, MD, FRCPC; Glen S. Hazlewood, MD, FRCPC; Pooneh Akhavan, MD, FRCPC; Orit Schieir, MSc; and Sanjay Dixit, MD, FRCPC
Download PDF
In this installment, we present the results of survey questions pertaining to treatment with traditional and biologic disease-modifying antirheumatic drugs (DMARDs).
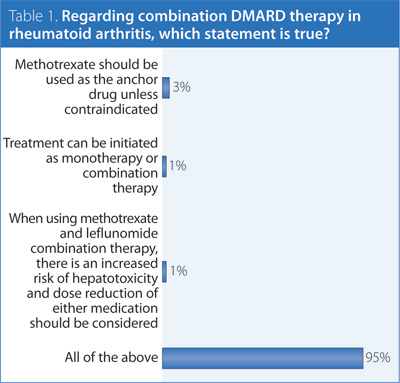
1. Regarding combination DMARD therapy in rheumatoid arthritis (RA), which statement is true?
Answer: All of the above.
Recommendation/supporting evidence: American College of Rheumatology (ACR) 2012,1 National Institute of Clinical Excellence (NICE) 2009,2 European League Against Rheumatism (EULAR) 2013,3 Visser 2009.4
Different highly rated guidelines came to different conclusions regarding the same literature. While the body of evidence supporting combination therapy has some limitations, there is sufficient evidence to consider the use of specific DMARD combinations as initial therapy and/or after inadequate response to monotherapy, particularly in patients with poor prognostic features, moderate-high disease activity, and recent-onset disease. There is sufficient evidence to support the use of methotrexate (MTX) as the anchor drug when using combination therapy, although other DMARD combinations may also be considered. Several different combination therapies have been shown to be effective in the treatment of RA, but direct comparative data of the effectiveness of different combinations is lacking. The choice of combination should be left to the discretion of the rheumatologist as a shared decision with the patient, based on individual patient circumstances. There is evidence from randomized controlled trials supporting the efficacy of MTX and leflunomide (LEF) in patients with high disease activity with an inadequate response to MTX. Many patients have been successfully treated with this combination without serious adverse events; however in general, other combination therapies of proven efficacy would be preferred over MTX + LEF due to increased gastrointestinal side effects and hepatoxicity. LEF combination therapy is typically considered after an inadequate response to MTX, and in this situation it is not desirable to withdraw MTX to treat with LEF as this may result in worsening of disease control. If MTX + LEF is used, liver enzymes should be monitored monthly and dose reduction of LEF (to 10 mg) or MTX should be considered. Similarly, clinicians should exercise caution when combining LEF with other drugs that have the potential to cause liver injury.
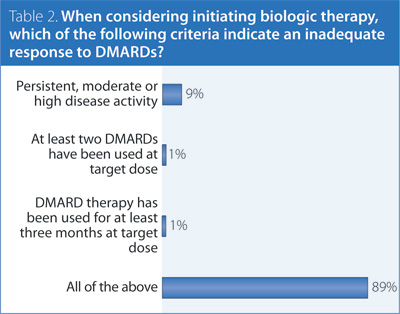
2. When considering initiating biologic therapy, which of the following criteria indicate an inadequate response to DMARDs?
Answer: All of the above.
Recommendation/supporting evidence: NICE 2009.2
Biologics, while proven effective in DMARD inadequate responders and DMARD-naïve patients, are associated with higher costs and potential risk for toxicity. Prior treatment with two DMARDs in monotherapy or combination therapy balances the potential opportunity for a response to DMARD therapy against the early initiation of a biologic which may be necessary to reach the treatment target. Three months at target dose is a sufficient period to observe a therapeutic effect for most DMARDs while minimizing delays in adjusting treatment.
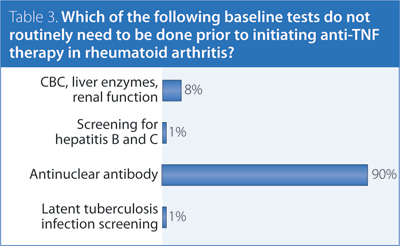
3. Which of the following baseline tests do not routinely need to be done prior to initiating anti-tumour necrosis factor (TNF) therapy in RA?
Answer: Antinuclear antibody (ANA).
Recommendation/supporting evidence: Australian Rheumatology Association (ARA) 2010,5 ACR 2012.1
Although there is only weak evidence supporting existing guidelines, the recommendations made by the ACR and ARA are reasonable. Having a baseline ANA assessment is not mandatory but could be considered, as it may be useful in patients who develop lupus-like symptoms. Based on expert opinion, there is insufficient evidence to recommend immunoglobulin screening or B-cell levels prior to rituximab. Investigations related to managing comorbidities or cardiovascular risk may also be necessary when treating with a biologic but are beyond the scope of the present guidelines.
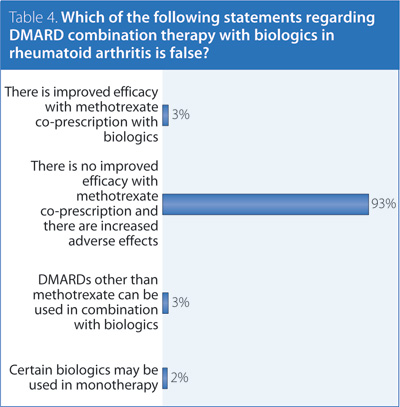
4. Which of the following statements regarding DMARD combination therapy with biologics in RA is false?
Answer: There is no improved efficacy with MTX co-prescription and there are increased side effects.
Recommendation/supporting evidence: EULAR 2013,3 Furst 2010,6 Fautrel 2010.7
There is strong evidence to recommend coprescription of MTX with biologic agents. In cases where MTX cannot be used, another DMARD is recommended. If coprescription with MTX or another DMARD is not possible, certain biologic agents may be used in monotherapy. Currently, etanercept, adalimumab, certolizumab, abatacept, and tocilizumab are licensed for use as monotherapy in Canada. Patients that have remained in a low disease-activity state taking biologic monotherapy may not require the reintroduction of a DMARD.
For further information on these recommendations and the supporting evidence of these results, please consult the CRA RA Guidelines document, available at www.rheum.ca/en/publications/cra_ra_guidelines.
References
1. Singh JA, Furst DE, Bharat A, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res 2012; 64(5):625-39.
2. National Institute of Clinical Excellence (NICE). Rheumatoid arthritis: The management of rheumatoid arthritis in adults: NICE clinical guidance 79; 2009. Available from: www.nice.org.uk/nicemedia/pdf/CG79NICEGuideline.pdf
3. Smolen JS, Landewé R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 2013. [Epub ahead of print]
4. Visser K, Katchamart W, Loza E, et al. Multinational evidence-based recommendations for the use of methotrexate in rheumatic disorders with a focus on rheumatoid arthritis: integrating systematic literature research and expert opinion of a broad international panel of rheumatologists in the 3E Initiative. Ann Rheum Dis 2009; 68(7):1086-93.
5. Australian Rheumatology Association (ARA). Updated recommendations for the use of biologic agents for the treatment of rheumatic diseases; 2010. Available from: www.rheumatology.org.au/otherpages/biological-guidelines.asp
6. Furst DE, Keystone EC, Fleischmann R, et al. Updated consensus statement on biological agents for the treatment of rheumatic diseases 2009. Ann Rheum Dis 2010; 69 Suppl 1:i2-29.
7. Fautrel B, Pham T, Mouterde G, et al. Recommendations of the French Society of Rheumatology regarding TNF alpha antagonist therapy in patients with rheumatoid arthritis. Joint Bone Spine 2007; 74(6):627-37.
Sankalp Bhavsar, MD, FRCPC
Rheumatology Fellow,
McMaster University
Hamilton, Ontario
on behalf of
Carter Thorne, MD, FRCPC, FACP;
Claire Bombardier, MD, FRCPC;
Vivian P. Bykerk, MD, FRCPC;
Glen S. Hazlewood, MD, FRCPC;
Pooneh Akhavan, MD, FRCPC;
Orit Schieir, MSc; and
Sanjay Dixit, MD, FRCPC |



